
 2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���| ���� | CH4 | CO2 | CO | H2 |
| ������� | 0.1 | 0.1 | 0.4 | 0.4 |
 2CO(g)��2H2(g) �ġ�H= ��
2CO(g)��2H2(g) �ġ�H= ��
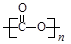 ��CO2+8e��+6H2O=CH4+8OH��
��CO2+8e��+6H2O=CH4+8OH�� �����x=4������K=
�����x=4������K=  =
= 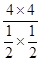 =64�����ɸ�˹���ɵá�H=(-890.3+2.8��2+566��2) kJ��mol��1=+247.3kJ��mol��1����2�����¶����ߣ��������Խ��͡���CO2(g)��CH4(g)
=64�����ɸ�˹���ɵá�H=(-890.3+2.8��2+566��2) kJ��mol��1=+247.3kJ��mol��1����2�����¶����ߣ��������Խ��͡���CO2(g)��CH4(g) CH3COOH(l)������ѹǿ�����������̼����Ũ�ȣ����������ת���ʡ���Cu2Al2O4��CuΪ+1�ۣ�Cu2Al2O4��������������ͭ����������NO��ˮ�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ����3����CO2�����Ӿ۷�Ӧʱ������C=O˫���е�
CH3COOH(l)������ѹǿ�����������̼����Ũ�ȣ����������ת���ʡ���Cu2Al2O4��CuΪ+1�ۣ�Cu2Al2O4��������������ͭ����������NO��ˮ�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ����3����CO2�����Ӿ۷�Ӧʱ������C=O˫���е� �����ۺϵ�
�����ۺϵ�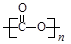 ����CO2��ͭ�缫�Ͽ�ת��Ϊ���飬C�Ļ��ϼ���+4��Ϊ-4��������ԭ��Ӧ���ɵ��Ӻ͵���غ㡢�����غ�д���缫��Ӧʽ��
����CO2��ͭ�缫�Ͽ�ת��Ϊ���飬C�Ļ��ϼ���+4��Ϊ-4��������ԭ��Ӧ���ɵ��Ӻ͵���غ㡢�����غ�д���缫��Ӧʽ��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CO(NH2)2(l)+H2O(l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
CO(NH2)2(l)+H2O(l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�| T / �� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
 ?2NH3(g) ��H2����92.4 kJ��mol��1
?2NH3(g) ��H2����92.4 kJ��mol��1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3 (g) ��H =" -92.4" kJ/mol
2NH3 (g) ��H =" -92.4" kJ/mol
| ��Ӧ | �����̵� | ��ҵ�̵� | ||||
| �¶�/�� | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84��10-31 | 0.1 | 5��108 | 1.847 | 0.507 | 0.152 |
 2NH3 (g)��ü�������H2��ת����Ϊ40%��
2NH3 (g)��ü�������H2��ת����Ϊ40%��| | N2 | H2 | NH3 |
| �� | 1 | 3 | 0 |
| �� | 0.5 | 1.5 | 1 |
| �� | 0 | 0 | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

 ��
�� ����ԭ
����ԭ �ķ���Ҳ�������������������Ⱦ�����磺
�ķ���Ҳ�������������������Ⱦ�����磺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO(NH2)2(l)��H2O(g)�ںϳ����н��С���ͼ�Т���������Ϊ�ϳ����а���ͬ��̼�� [n(NH3)/n(CO2)]��ˮ̼��[n(H2O)/n(CO2)]Ͷ��ʱ������̼ת���ʵ������
CO(NH2)2(l)��H2O(g)�ںϳ����н��С���ͼ�Т���������Ϊ�ϳ����а���ͬ��̼�� [n(NH3)/n(CO2)]��ˮ̼��[n(H2O)/n(CO2)]Ͷ��ʱ������̼ת���ʵ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 CH3OH(g)
CH3OH(g)
| A�������¶� |
| B����CH3OH(g)����ϵ�з��� |
| C������He��ʹ��ϵ��ѹǿ���� |
| D���ٳ���1 mol CO��2 mol H2 |

 BaCO3(s)+SO42��(aq)��
BaCO3(s)+SO42��(aq)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| | �� | �� | Na2CO3 | ���ʯ | ʯī |
| �۵㣨�棩 | 63.65 | 97.8 | 851 | 3550 | 3850 |
| �е㣨�棩 | 774 | 882.9 | 1850���ֽ����CO2�� | ---- | 4250 |
 2 Na2CO3��l��+ C(s�����ʯ) ��H=��1080.9kJ/mol
2 Na2CO3��l��+ C(s�����ʯ) ��H=��1080.9kJ/mol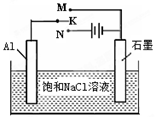
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��+184.6kJ��mol-1 | B���D92.3kJ��mol-1 |
| C��+92.3kJ | D��+92.3kJ��mol-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com