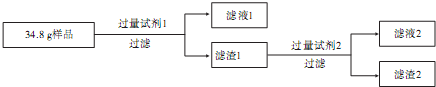
��������1��������Ӧ��CO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g��������ͼ1��֪��0.5mol CO
2��1.5mol H
2ת���ʴ�80%ʱ����23kJ-3.4kJ=19.6kJ������1mol������̼�����ų�������ȷ����H����д�÷�Ӧ���Ȼ�ѧ����ʽ��
��2���ٸ���v=
����v��H
2����
���¶�һ��������II����ƽ��ʱ��ʱ�����̣�ƽ��ʱ���������ʵ�����С��˵���ı���������Ӧ���ʣ���ƽ��������Ӧ�����ƶ�����������Ϊ���������£�Ӧ�����������̼��Ũ�ȣ�
I��II��ѧƽ�ⳣ����ͬ��ƽ�ⳣ��K=
=
| n(CH3OH)?n(H2O) |
| n(CO2)?[n(H2)]3 |
��V2������������䣬����
| n(CH3OH)?n(H2O) |
| n(CO2)?[n(H3)]3 |
��ȼ���ƽ��ʱ������̼���ʵ�����������������IIƽ��ʱc��CO
2����
�۵���Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�ȡ��ٷֺ������䣬�Լ��ɴ�������һЩ��Ҳ�������仯��ע��ѡ���жϵ���������Ӧ���ŷ�Ӧ�Ľ��з����仯�������������ɱ仯����ֵʱ��˵�����淴Ӧ����ƽ��״̬��
��3������ͼ��֪���¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ�
���Ƚ�Cu
2Al
2O
4������������ʽ��Cu
2O?Al
2O
3���ٸ������������ᷴӦ�������ӷ���ʽ��ע��һ��ͭ���л�ԭ�ԣ������ᷴӦ������ԭ��Ӧ��NO���ɣ�
��4���ٶ�����̼Ϊ�������壬Li
2O��Na
2O��MgO��������CO
2���������أ�
����500�棬CO
2��Li
4SiO
4�Ӵ�������Li
2CO
3����Ӧ��ΪCO
2��Li
4SiO
4����������Li
2CO
3�����������غ��֪���ﻹ��Li
2SiO
3��
���
�⣺��1������ͼ1��֪��0.5mol CO
2��1.5mol H
2ת���ʴ�80%ʱ����23kJ-3.4kJ=19.6kJ��1mol������̼��Ӧ�ų�������Ϊ
��19.6kJ=49kJ����÷�Ӧ���Ȼ�ѧ����ʽ��CO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g����H=-49kJ?mol
-1��
�ʴ�Ϊ��CO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g����H=-49 kJ?mol
-1��
��2������ͼ��֪��ƽ��ʱ�μӷ�Ӧ����Ϊ2mol-1mol=1mol����v��H
2��=
=0.05mol/��L?min����
�ʴ�Ϊ��0.05mol/��L?min����
���¶�һ��������II����ƽ��ʱ��ʱ�����̣�ƽ��ʱ���������ʵ�����С��˵���ı���������Ӧ���ʣ���ƽ��������Ӧ�����ƶ�����������Ϊ���������£�Ӧ�����������̼��Ũ�ȣ�
ƽ�ⳣ��K=
=
| n(CH3OH)?n(H2O) |
| n(CO2)?[n(H2)]3 |
��V2������������䣬����
| n(CH3OH)?n(H2O) |
| n(CO2)?[n(H3)]3 |
��ȣ�
��Ӧ����I��CO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g��
��ʼ��mol����1 2 0 0
ת����mol����
1
ƽ�⣨mol����
1
��Ӧ����II��CO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g��
��ʼ��mol����2 0 0
ת����mol����2-0.5 0.5 0.5
ƽ�⣨mol����0.5 0.5 0.5
��
=
�����n��CO
2��=12����ƽ��ʱc��CO
2��=
=2.4mol/L��
�ʴ�Ϊ�����������̼��Ũ�ȣ�2.4mol/L��
��A���淴Ӧ���л�����������ʵ�����С�����������䣬���淴Ӧ����ƽ����Է���������С����������ƽ����Է�����������ʱ��ı䣬˵����Ӧ����ƽ�⣬��A��ȷ��
B����v��H
2��
����v��CH
3OH��
��=3��1ʱ����Ӧ����ƽ�⣬��3v��H
2��
��=v��CH
3OH��
�����ɵ�v��H
2��
����v��CH
3OH��
��=1��3��˵���淴Ӧ���ʽϴ�ƽ�����淴Ӧ���У���B����
C������������䣬�淴Ӧ���л�����������ʵ�����С����������ѹǿ��С�����������ѹǿ����ʱ��ı䣬˵����Ӧ����ƽ�⣬��C��ȷ��
D����λʱ��������CH
3OH��H
2O�����ʵ�����ͬ������ʾ����Ӧ���ʣ���Ӧʼ�հ�1��1���У�����˵������ƽ�⣬��D����
�ʴ�Ϊ��AC��
��3�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ������¶����߶�������������ʽ��ͣ�
�ʴ�Ϊ���¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ�
��Cu
2Al
2O
4������������ʽ��Cu
2O?Al
2O
3��һ��ͭ���л�ԭ�ԣ������ᷴӦ������ԭ��Ӧ��NO���ɣ������ᷴӦ�������ӷ���ʽ��3Cu
2Al
2O
4+32H
++2NO
3-=6Cu
2++6Al
3++2NO��+16H
2O��
�ʴ�Ϊ��3Cu
2Al
2O
4+32H
++2NO
3-=6Cu
2++6Al
3++2NO��+16H
2O��
��4����a��Li
2O��Na
2O��MgO�����ڼ��������������������������CO
2�����ڼ�����������Ѱ������CO
2���������ʣ���a��ȷ��
b��Li
2O��Na
2O��MgO��������CO
2���ơ�þΪ��A����A��Ԫ�أ����Կ��ڢ�A����A��Ԫ���γɵ���������Ѱ������CO
2���������ʣ���b��ȷ��
c��Li
2O��Na
2O��MgO��������CO
2�������Ƕ�û��ǿ�����ԣ������ն�����̼��������ԭ�أ���c����
�ʴ�Ϊ��ab��
����500�棬CO
2��Li
4SiO
4�Ӵ�������Li
2CO
3����Ӧ��ΪCO
2��Li
4SiO
4����������Li
2CO
3�����������غ��֪���ﻹ��Li
2SiO
3�����Ի�ѧ����ʽΪ��CO
2+Li
4SiO
4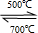
Li
2CO
3+Li
2SiO
3��
�ʴ�Ϊ��CO
2+Li
4SiO
4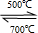
Li
2CO
3+Li
2SiO
3��


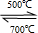 Li2CO3+Li2SiO3��
Li2CO3+Li2SiO3�� Li2CO3+Li2SiO3��
Li2CO3+Li2SiO3��