
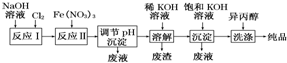
| A�� | ��Ӧ����ҪΪ2NaOH+Cl2�TNaCl+NaClO+H2O ��Ӧ������ӷ���ʽΪ3ClO-+10OH-+2Fe3+�T2FeO42-+3Cl-+5H2O | |
| B�� | ���뱥��KOH��Һ��Ŀ��������K+Ũ�ȣ��ٽ�K2FeO4�������� | |
| C�� | ����pH�����ij���Ϊ�������ƣ��������ϴ�ӵ���ҪĿ���������ڲ�Ʒ���� | |
| D�� | ���������һ�������ˮ���������䴦��ˮ��ԭ��Ϊ���������ǿ�����ԣ���ɱ��������������Fe��OH��3�������ԣ����������� |
���� ���ݷ�Ӧ���̿�֪����Ӧ��Ϊ��2NaOH+Cl2�TNaCl+NaClO+H2O����Ӧ��3ClO-+10 OH-+2Fe3+=2FeO42-+3Cl-+5H2O��������ҺpH��ʹ��ҺFe3+��FeO42-ת��Ϊ����������ϡKOH��Һ�ܽ⣬���˳�ȥ�����������ټ��뱥��KOH��Һ��������K+Ũ�ȣ��ٽ�K2FeO4�����������ñ���ϴ�Ӽȿɳ�ȥ�����������ֿɼ�СK2FeO4����ʧ���ݴ˴��⣮
��� �⣺���ݷ�Ӧ���̿�֪����Ӧ��Ϊ��2NaOH+Cl2�TNaCl+NaClO+H2O����Ӧ��3ClO-+10 OH-+2Fe3+=2FeO42-+3Cl-+5H2O��������ҺpH��ʹ��ҺFe3+��FeO42-ת��Ϊ����������ϡKOH��Һ�ܽ⣬���˳�ȥ�����������ټ��뱥��KOH��Һ��������K+Ũ�ȣ��ٽ�K2FeO4�����������ñ���ϴ�Ӽȿɳ�ȥ�����������ֿɼ�СK2FeO4����ʧ��
A����������ķ�����֪����Ӧ��Ϊ��2NaOH+Cl2�TNaCl+NaClO+H2O����Ӧ��3ClO-+10 OH-+2Fe3+=2FeO42-+3Cl-+5H2O����A��ȷ��
B����������ķ�����֪�����뱥��KOH��Һ��Ŀ��������K+Ũ�ȣ��ٽ�K2FeO4������������B��ȷ��
C������pH�����ij���Ϊ�����������������ϴ�ӵ���ҪĿ���Ǽ�СK2FeO4����ʧ����C����
D�����������һ�������ˮ���������䴦��ˮ��ԭ��Ϊ���������ǿ�����ԣ���ɱ��������ͬʱ�ܱ���ԭ�ɵ�Fe��OH��3���壬�������ԣ����������ã�ʹˮ�е����������ʳ�������D��ȷ��
��ѡC��
���� ���⿼��ʵ���Ʊ���������ȷ��������ԭ���ǽ���ؼ���ע�����Ŀ���̵ķ��������֪ʶ�л�����������ϺõĿ���ѧ���ۺ��������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2S��Һ��c��Na+����c��S2-����c��HS-����c��OH-����c��H2S�� | |
| B�� | Na2CO3��Һ��c��Na+��+c��H+��=c��CO${\;}_{3}^{2-}$��+c��HCO${\;}_{3}^{-}$��+c��OH-�� | |
| C�� | Na2C2O4��Һ��c��OH-��=c��H+��+c��HC2O${\;}_{4}^{-}$��+2c��H2C2O4�� | |
| D�� | CH3COONa��CaCl2�����Һ��c��Na+��+c��Ca2+��=c��CH3COO-��+c��CH3COOH��+2c��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
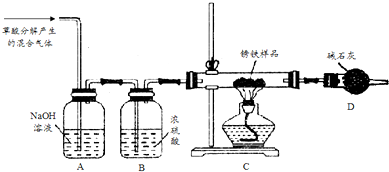
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
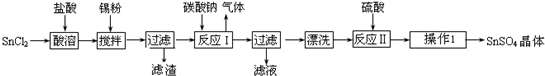
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2��H2O | B�� | BeCl2��BF3 | C�� | CH4��NH3 | D�� | C2H2��C2H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
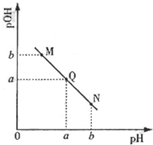 ��֪��25��ʱ��CH3COOH��NH3•H2O�ĵ��볣����ȣ�
��֪��25��ʱ��CH3COOH��NH3•H2O�ĵ��볣����ȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʪ��pH��ֽ��ϡ����Һ��pH���ⶨֵƫС | |
| B�� | ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫ�� | |
| C�� | �ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС | |
| D�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ֵƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2SO4 | B�� | NaOH | C�� | NaCl | D�� | AgNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ | B�� | ���� | C�� | ���� | D�� | ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com