
1Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH4+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��Al(OH)4-(��AlO2-) |
��1��Ag+��Mg2+��Fe3+��
��2��CO32- �� Al(OH)4-����AlO2-��
��3��NH4++OH-=NH3. H2O
��4��n(H+): n(NH4+):n(Al3+)=2:3:1
��2Fe3++2I-= I2+2Fe2+
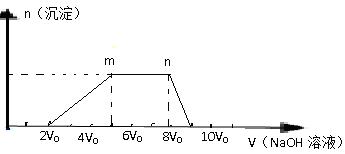
���������������1��������Ŀ����ͼ������NaOH��Һ���������ӣ����ɳ���Ȼ��������ȫ�ܽ⣬˵��ԭ��Һ����Al3+����������ȫ�ܽ⣬˵������Һ����Ag+��Mg2+��Fe3+��
��2��Al3+����CO32- �� Al(OH)4-��Ӧ����������������һ�������ڡ�
��3��m��n�γ������仯��ΪNaOH��NH4+��Ӧ�����ӷ���ʽΪ��NH4++OH-=NH3.H2O��
��4������ͼ�����߿�֪����H+��Ӧ���ĵ�NaOH��Һ���Ϊ2V0����n(H+)="2" V0c(NaOH)����Al3+��Ӧ����Al(OH)3��������NaOH��Һ3V0����n(Al3+)= V0c(NaOH)����NH4+��Ӧ���ĵ�NaOH��Һ�����Ϊ3V0����n(NH4+)="3" V0c(NaOH)������n(H+): n(NH4+):n(Al3+)="2" V0c(NaOH)��3 V0c(NaOH)��V0c(NaOH)=2:3:1��
��Fe3+�������Դ���I2������Fe3+����I?����ΪI2�����ӷ���ʽΪ��2Fe3++2I-= I2+2Fe2+��
���㣺���⿼��ͼ��ķ�������Һ�����ӵ��жϡ����ӷ���ʽ���жϼ���ؼ��㡣


 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.��4�֣�ʵ������һƿ�������Һ��ʵ����Աȷ�����п��ܺ���NH4+��K+��Na+��Mg2+��Ba2+��Al3+��Fe3+��Cl-��I-��NO3-��CO32-��SO42-��ȡ����Һ��������ʵ�飺
��ȡpH��ֽ���飬������Һ��ǿ���ԡ�
��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ��
����ȡ������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ������������
��ȡ��������������Һ��Na2CO3��Һ���а�ɫ�������ɡ�
�ݽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
����ڵ�ˮ���м���HNO3�ữ��AgNO3��Һ�а�ɫ������
��������ʵ����ʵȷ���������жϸ���Һ��
��1���϶����ڵ������� ��
��2������ȷ���Ƿ���ڵ������� ��
��. ��6�֣���������(SeO2)��һ�����������䱻��ԭ��ĵ��������ܳ�Ϊ������Ⱦ�ͨ����ŨHNO3��ŨH2SO4��Ӧ����SeO2�Ի���Se�����������գ�
��1��Se��ŨHNO3��Ӧ�Ļ�ԭ����ΪNO��NO2����NO��NO2�����ʵ���֮��Ϊ1��1��д��Se��ŨHNO3�ķ�Ӧ����ʽ ��
��2����֪��Se+2H2SO4(Ũ)��2SO2��+SeO2+2H2O��2SO2+SeO2+2H2O��Se+2SO42-+4H+
SeO2��H2SO4(Ũ)��SO2����������ǿ������˳���� ��
��3�����յõ���SeO2�ĺ���������ͨ������ķ����ⶨ��
��SeO2+ 4KI+ 4HNO3��Se+2I2+ 4KNO3+2H2O ��I2+2Na2S2O3��Na2S4O6+2NaI
ʵ���У�ȷ����SeO2��Ʒ0.1500g��������0.2000 mol/L��Na2S2O3��Һ25.00 mL�����ⶨ����Ʒ��SeO2����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����з�Ӧ�����ӷ���ʽ,����Ӧ�Ļ�ѧ����ʽ��ÿ��2��7С�14�֣�
��1��ʯ��ʯ����ϡ���� ��
��2��ϡ����������������Һ�ķ�Ӧ ��
��3��̼��������Һ�����ᷴӦ ��
��4��������ͭ��ϡ���ᷴӦ ��
��5��2H++ CO32-��CO2��+H2O ��
��6��Cu��2Ag����Cu2����2Ag ��
��7��CO2+2OH-��CO32-+H2O ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����¼������ʢٸ����ʳ�ξ��� �ڱ����� ��ˮ�� ������ ��KNO3��Һ
���������ܵ������ ���������������ڵ���ʵ���
�������������ڷǵ���ʵ��� ������ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)(4��)д������������ˮ��Һ�еĵ��뷽��ʽ��
HNO3 Ba(OH)2
NaHCO3 NaHSO4
(2)(4��)��ʵ�����Ʊ������Ĺ����У����������ͨ����������������Һ�������գ���д���˷�Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ��
�û�ѧ��Ӧ�У��������� ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ӵ�ʳ����ͨ���������KIO3�������������������±��ֳ���ǿ�������ԣ����Ժ͵⻯��������εȻ�ԭ�����ʷ�����Ӧ��
(1)д��KIO3��KI��ϡ��������з�����Ӧ�����ӷ���ʽ______________________
(2)Ϊ�ⶨ�˼ӵ����е�Ԫ�صĺ�����ijѧ�������������ʵ�飺
A��ȷ��ȡwgʳ�Σ�ʹ����ȫ�ܽ�������������ˮ�У�
B����ϡ�����ữ������Һ�����������KI��Һ��ʹ���ַ�Ӧ��
C����___________________Ϊָʾ�����˵μ������ʵ���Ũ��Ϊ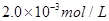 �������������Һ10.0mL��ǡ����ȫ��Ӧ���жϷ�Ӧ��ȫ��ʵ������Ϊ____________________________________,��üӵ�����Ʒ�е�Ԫ�صĺ���Ϊ______________________mg/Kg(�ú�w�Ĵ���ʽ��ʾ)��
�������������Һ10.0mL��ǡ����ȫ��Ӧ���жϷ�Ӧ��ȫ��ʵ������Ϊ____________________________________,��üӵ�����Ʒ�е�Ԫ�صĺ���Ϊ______________________mg/Kg(�ú�w�Ĵ���ʽ��ʾ)��
����֪��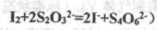 ��
��
(3)ijѧ�����ֽ���������ʵ�飺
A��ȷ��ȡ1.0g������NaCl����3mL����ˮ�����Һ����ҺΪ��ɫ��
B������5��ָʾ����1mL 0.lmol/L Kl��Һ���������Һ���仯��
C�������μ�l��1mol/L��������Һ���������Һ����ɫ
�Ʋ�ʵ���в�����ɫ�����ԭ�������ӷ���ʽ��ʾ________________________
����ѧ���ҵ�ʵ���������ѧ����ʵ�������з�________________________��ƫ��ƫС����ȷ������ԭ����________________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Cr2O72-��CN-�ǹ�ҵ��ˮ�г�������Ⱦ��������������̿ɶԷֱ��������ӵķ�ˮ�ۺϴ�����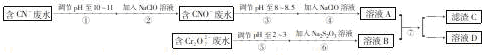
��֪��Cr3+Ҳ��һ�����ԣ�Cr3+��pH=6.0ʱ��ʼ������pH=8.0ʱ������ȫ��
�ش��������⣺
��1����֪����������������ɣ�д����Ӧ�����ӷ���ʽ ���������NaClO�ɽ�CNO- ����Ϊ��ȫ������Һ���ù��������������IJ�֧��ȼ�յ����������д������������Ļ�ѧʽ ��
��2�� ����ķ�ӦΪ S2O32-+ Cr2O72-+2H+��SO42-+ Cr3++H2O(δ��ƽ)��ÿ����1mol Cr2O72-ת�� mole-��
��3�� ����C�Ļ�ѧʽΪCr(OH)3����ҺA����ҺB��Ͽ�ʹ��ˮ�е�Cr3+������ԭ���� ��
��4������ߵIJ�������Ϊ �����ò�����ʵ�����н��У�������ĵIJ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������������ԭ��ӦҲ�����ȷ�Ӧ����( )
| A�����ȵ�̿�������̼��Ӧ | B������ϡ����ķ�Ӧ |
| C��������������ķ�ĩ���Ȼ�茶����� | D��������Ʒ����ķ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com