
·ÖĪö £Ø1£©356 g CH4•9H2OæÉŅŌŹĶ·Å³ö2 mol CH4£¬¼ĘĖć¼×ĶéČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼ÓėĘųĢ¬Ė®·Å³öµÄČČĮ棬ŌŁ¼ĘĖćĘųĢ¬Ė®×Ŗ»ÆĪŖŅŗĢ¬Ė®·Å³öµÄČČĮ棬Į½²æ·ÖÖ®ŗĶ¾łĪŖČ¼ÉÕÉś³ÉŅŗĢ¬Ė®·Å³öµÄČČĮ棻
£Ø2£©¼ĘĖć1molĘųĢ¬ŅŅÅšĶéŌŚŃõĘųÖŠČ¼ÉÕÉś³É¹ĢĢ¬ČżŃõ»Æ¶žÅšŗĶŅŗĢ¬Ė®·Å³öµÄČČĮ棬עĆ÷ĪļÖŹµÄ¾Ū¼ÆדĢ¬Óė·“Ó¦Čȏ銓ČČ»Æѧ·½³ĢŹ½£»
£Ø3£©1g¶”ĶéĘųĢåĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶH2O£Øl£©·Å³öČČĮæ50 kJ£¬Ōņ1mol¶”Ķ鷓Ӧɜ³ÉCO2ŗĶH2O£Øl£©·Å³öČČĮæĪŖ50kJ”Į$\frac{1mol”Į58g/mol}{1g}$=2900kJ£¬×¢Ć÷ĪļÖŹµÄ¾Ū¼ÆדĢ¬Óė·“Ó¦Čȏ銓ČČ»Æѧ·½³ĢŹ½£»
£Ø4£©¢ŁÓŠŠ§”°¼õĢ¼”±µÄŹÖ¶ĪÖ®Ņ»ŹĒ½ŚÄÜ£¬ĄūÓĆĢ«Ńō¹ā“߻ƷֽāĖ®ÖĘĒāŹĒ×ī½ŚÄܵģ¬¼õÉŁµēÄÜ”¢ĢģČ»Ęų”¢ČČÄܵĥūÓĆ£»
¢ŚĄūÓĆŅŃÖŖČČ»Æѧ·½³ĢŹ½³ĖŅŌŹŹµ±µÄĻµŹż½ųŠŠ¼Ó¼õ¹¹ŌģÄæ±źČČ»Æѧ·½³ĢŹ½£®
½ā“š ½ā£ŗ£Ø1£©356 g CH4•9H2OĪļÖŹµÄĮæĪŖ$\frac{365g}{£Ø16+18”Į9£©g/mol}$=2mol£¬æÉŅŌŹĶ·Å³ö2 mol CH4£¬ÓÉCH4£Øg£©+2O2£Øg£©ØTCO2£Øg£©+2H2O£Øg£©”÷H=-802.3kJ•mol-1£¬æÉÖŖ2mol¼×ĶéČ¼ÉÕÉś³ÉĘųĢ¬Ė®·Å³öČČĮæĪŖ2mol”Į802.3kJ/mol=1604.6kJ£¬Ķ¬Ź±Éś³É4molH2O£Øg£©£¬ÓÉH2O£Øl£©ØTH2O£Øg£©”÷H=+44kJ•mol-1£¬æÉÖŖ4molH2O£Øg£©×Ŗ»ÆĪŖ4molH2O£Øl£©·Å³öµÄČČĮæĪŖ4mol”Į44kJ/mol=176kJ£¬¹Ź·Å³öµÄČČĮæĪŖ1604.6kJ+176kJ=1 780.6 kJ£»
¹Ź“š°øĪŖ£ŗ1780.6£»
£Ø2£©1molĘųĢ¬øßÄÜČ¼ĮĻŅŅÅšĶéŌŚŃõĘųÖŠČ¼ÉÕ£¬Éś³É¹ĢĢ¬ČżŃõ»Æ¶žÅšŗĶŅŗĢ¬Ė®£¬·Å³öµÄČČĮæĪŖ649.5kJ”Į$\frac{1mol}{0.3mol}$=2165kJ£¬·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗB2H6£Øg£©+3O2£Øg£©ØTB2O3£Øs£©+3H2O£Øl£©”÷H=-2165kJ/mol£»
¹Ź“š°øĪŖ£ŗB2H6£Øg£©+3O2£Øg£©ØTB2O3£Øs£©+3H2O£Øl£©”÷H=-2165 kJ/mol£»
£Ø3£©1g¶”ĶéĘųĢåĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶH2O£Øl£©·Å³öČČĮæ50 kJ£¬Ōņ1mol¶”Ķ鷓Ӧɜ³ÉCO2ŗĶH2O£Øl£©·Å³öČČĮæĪŖ50kJ”Į$\frac{1mol”Į58g/mol}{1g}$=2900kJ£¬¶”ĶéČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗC4H10£Øg£©+$\frac{13}{2}$O2£Øg£©ØT4CO2£Øg£©+5H2O£Øl£©”÷H=-2900 kJ/mol£¬
¹Ź“š°øĪŖ£ŗC4H10£Øg£©+$\frac{13}{2}$O2£Øg£©ØT4CO2£Øg£©+5H2O£Øl£©”÷H=-2900 kJ/mol£»
£Ø4£©¢ŁA£®ĻūŗĵēÄÜ£¬²»ŹĒ×ī½ŚÄܵķ½·Ø£¬¹ŹA“ķĪó£»
B£®ĻūŗÄČČÄÜ£¬²»ŹĒ¼õĢ¼µÄŹÖ¶Ī£¬¹ŹB“ķĪó£»
C£®ĄūÓĆĢ«Ńō¹ā“߻ƷֽāĖ®ÖĘĒāŹĒ×ī½ŚÄܵģ¬¹ŹCÕżČ·£»
D£®ĢģČ»ĘųŹĒ·ĒŌŁÉśÄÜŌ“£¬ĒŅĻūŗÄČČÄÜ£¬²»ŹĒ×ī½ŚÄܵķ½·Ø£¬¹ŹD“ķĪó£®
¹ŹŃ”C£»
¢ŚŅŃÖŖ£ŗ¢ń£®CO£Øg£©+2H2£Øg£©?CH3OH£Øg£©”÷H=-90.7kJ•mol-1
¢ņ£®2CH3OH£Øg£©?CH3OCH3£Øg£©+H2O£Øg£©”÷H=-23.5kJ•mol-1
¢ó£®CO£Øg£©+H2O£Øg£©?CO2£Øg£©+H2£Øg£©”÷H=-41.2kJ•mol-1
øł¾ŻøĒĖ¹¶ØĀÉ£¬¢ń”Į2+¢ņ-¢ó”Į2æÉµĆ£ŗ2CO2£Øg£©+6H2£Øg£©=CH3OCH3£Øg£©+3H2O£Øg£©”÷H=-122.5 kJ•mol-1£¬
¹Ź“š°øĪŖ£ŗ2CO2£Øg£©+6H2£Øg£©=CH3OCH3£Øg£©+3H2O£Øg£©”÷H=-122.5 kJ•mol-1£®
µćĘĄ ±¾Ģā漲鷓ӦČČ¼ĘĖć”¢ČČ»Æѧ·½³ĢŹéŠ“µČ£¬×¢Ņā¶ŌøĒĖ¹¶ØĀɵĥķ½āÓėÓ¦ÓĆ£¬·“Ó¦ČČ¼ĘĖć³£Éę¼°£ŗČČ»Æѧ·½³ĢŹ½ÓėøĒĖ¹¶ØĀÉÓ¦ÓĆ”¢Č¼ÉÕČČÓėÖŠŗĶČČøÅÄīµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| “ĪŹż | 1 | 2 | 3 |
| µĪ¶ØĢå»ż/mL | 19.98 | 20.02 | 19.00 |
| ŹµŃ鱹ŗÅ | ŹµŃé²Ł×÷ | ĻÖĻó |
| 1 | Ļņ10mL 3mol/L KNO3ĖįŠŌČÜŅŗ£ØpH=1£©ÖŠ²åČėŅ»øł½ą¾»µÄAgĖ棬²¢µĪ¼ÓNaClČÜŅŗ | ĪŽ°×É«³ĮµķÉś³É |
| 2 | Ļņ10mL 1mol/L AgNO3ČÜŅŗÖŠµĪ¼Ó2mL 0.1mol/L FeSO4ČÜŅŗ£¬Õńµ“£¬ŌŁµĪ¼ÓĖįŠŌKMnO4ČÜŅŗ | ×ĻŗģÉ«²»ĶŹČ„ |
| 3 | Ļņ10mL 1mol/L Fe£ØNO3£©3ĖįŠŌČÜŅŗ£ØpH=1£©ÖŠ²åČėŅ»øł½ą¾»µÄAgĖ棬²¢µĪ¼ÓNaClČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅ“¼ŌŚĶ×÷“߻ƼĮµÄĢõ¼žĻĀ¼ÓČČŗĶæÕĘųµÄ·“Ó¦ | |
| B£® | ŅŅĻ©ĶØČėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗÖŠµÄ·“Ó¦ | |
| C£® | ¼×ĶéŌŚ¹āÕÕĢõ¼žĻĀÓėĀČĘų·¢ÉśµÄ·“Ó¦ | |
| D£® | ±½ÓėŅŗäåŌŚĢś·Ū×÷“߻ƼĮµÄĢõ¼žĻĀ·¢ÉśµÄ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Al”¢Cu”¢AgNO3 | B£® | Na2O2”¢Na2SO3”¢BaCl2 | ||
| C£® | CaCO3”¢Na2SiO3”¢CH3COONa | D£® | Ba£ØNO3£©2”¢Fe£ØOH£©2”¢NaOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ėę×ŵē×Ó²ćŹżŌö¶ą£¬¼ī½šŹōµÄŌ×Ó°ė¾¶Öš½„Ōö“ó | |
| B£® | ¼ī½šŹō¾ßÓŠĒ滹ŌŠŌ£¬ĖüĆĒµÄĄė×Ó¾ßÓŠĒæŃõ»ÆŠŌ | |
| C£® | ¼ī½šŹōµ„ÖŹµÄČŪ·ŠµćĖę×ÅŗĖµēŗÉŹżµÄŌö“ó¶ų½µµĶ | |
| D£® | ¼ī½šŹōŌŖĖŲŌŚ×ŌČ»½ēÖŠ¶¼ŹĒŅŌ»ÆŗĻĢ¬“ęŌŚµÄ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ū¢Ü¢Ż¢ą | B£® | ¢Ü¢Ż¢ß | C£® | ¢Ū¢Ż¢ß | D£® | ¢Ū¢Ü¢Ż¢ß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃé²Ł×÷ | ĻÖĻó¼°½įĀŪ | |
| ¢ń | “ņæŖ»īČūa£¬µĪ¼ÓĀČĖ®£¬¹Ų±Õ»īČū | AÖŠČÜŅŗ±äĪŖŗģ×ŲÉ« |
| ¢ņ | “µČėČČæÕĘų | AÖŠŗģ×ŲÉ«Ć÷ĻŌ±äĒ³£»BÖŠÓŠĘųÅŻ£¬²śÉś“óĮæ°×É«³Įµķ£¬»ģŗĻŅŗŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ |
| ¢ó | Ķ£Ö¹“µČėæÕĘų£¬“ņæŖ»īČūb£¬ÖšµĪ¼ÓČėH2O2ČÜŅŗ | æŖŹ¼Ź±ŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ£»¼ĢŠųµĪ¼ÓH2O2ČÜŅŗ£¬Ņ»¶ĪŹ±¼äŗ󣬻ģŗĻŅŗÖš½„±ä³Éŗģ×ŲÉ« |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
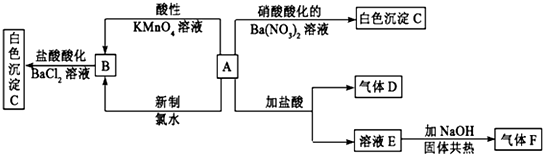
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | CuO×÷»¹Ō¼Į | B£® | CuO×÷Ńõ»Æ¼Į | ||
| C£® | ĶŌŖĖŲ»ÆŗĻ¼Ū½µµĶ | D£® | ĒāŌŖĖŲ»ÆŗĻ¼ŪÉżøß |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com