
ĻĀĮŠČÜŅŗÖŠ£¬Ąė×ÓÅØ¶Č“óŠ”µÄÅÅĮŠĖ³Šņ“ķĪóµÄŹĒ
A£®0.1mol/LĀČ»Æļ§ČÜŅŗÖŠ£ŗc(Cl£)£¾c(NH4+)£¾c(H+)£¾c(OH£)
B£®0.1mol/LĢ¼ĖįĒāÄĘČÜŅŗÖŠ£ŗ c(Na+)>c(HCO3£)>c(OH£)>c(CO32£)>c(H+)
C£®pH=2ŃĪĖįÓėpH=12°±Ė®µČĢå»ż»ģŗĻ£ŗc(Cl£)£¾c(NH4+)£¾c(H+)£¾c(OH£)
D£®0.2 mol/LCH3COOKŗĶHClµČĢå»ż»ģŗĻ£ŗc(CH3COO£)£¾c(Cl£)£¾c(CH3COOH)£¾c(H+)

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
0.5 L mol”¤L£1 FeCl3ČÜŅŗÓė0.2 L mol”¤L£1 KClČÜŅŗÖŠµÄCl£µÄŹżÄæÖ®±ČĪŖ£Ø £©”£
A£®5”Ć2 B£®3”Ć1 C£®15”Ć2 D£®1”Ć3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆ2mol/LµÄNaOHČÜŅŗµĪ¶ØpH=5µÄHCNČÜŅŗ100mLÖĮÖŠŠŌ£¬“ĖŹ±ČÜŅŗÖŠø÷Ąė×ÓÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ
A. c(Na£«)>c(CN£)>c(OH£)>c(H£«) B£®c(CN£)>c(Na£«)>c(H£«)> c(OH£)
C. c(Na£«)+c(CN£)=2mol”¤L£1 D£®c(Na£«)+c(H+)= c(CN£)+c(OH£)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ1LČÜŅŗÖŠ¼ŗµēĄėµÄĖ®ŹĒ10£3mol£¬ŌŚ“ĖČÜŅŗÖŠæÉÄÜ“óĮæ¹²“ęµÄĄė×Ó×éŹĒ£ŗ
¢ŁK£«”¢Na+”¢NO3£”¢SO42££»¢ŚCO32£”¢Cl£”¢Na+”¢K+£»¢ŪMg2+”¢NO3£”¢K+”¢Cl££»¢ÜNO3£”¢NH4+”¢SO42£”¢K+£»¢ŻFe2+”¢Cl£”¢SO42£”¢NO3££»¢ŽS2£”¢K+”¢Cl£”¢ClO£
A. ¢Ł¢Ś¢Ū¢Ü¢Ż¢Ž B. ¢Ł¢Ś¢Ū¢Ü C. ¢Ł¢Ż¢Ž D. ¢Ł
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌijĖįŠŌČÜŅŗ£ØæÉÄÜŗ¬ÓŠBr££¬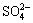 £¬H2SO3£¬
£¬H2SO3£¬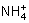 £©·Ö±š½ųŠŠČēĻĀŹµŃé£ŗ
£©·Ö±š½ųŠŠČēĻĀŹµŃé£ŗ
¢Ł ¼ÓČČŹ±·Å³öµÄĘųĢåæÉŅŌŹ¹Ę·ŗģČÜŅŗĶŹÉ«£»¢Ś ¼Ó¼īµ÷ÖĮ¼īŠŌŗ󣬼ÓČČŹ±·Å³öµÄĘųĢåæÉŅŌŹ¹ČóŹŖµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£»¢Ū ¼ÓČėĀČĖ®Ź±£¬ČÜŅŗĀŌĻŌ»ĘÉ«£¬ŌŁ¼ÓČėBaCl2ČÜŅŗŹ±£¬²śÉśµÄ°×É«³Įµķ²»ČÜÓŚĻ”ĻõĖį”£¶ŌÓŚĻĀĮŠĪļÖŹ²»ÄÜČ·ČĻĘäŌŚČÜŅŗÖŠŹĒ·ń“ęŌŚµÄŹĒ
A. Br£ B. 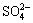 C. H2SO3 D. NH4+
C. H2SO3 D. NH4+
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éĄė×ÓŅ»¶ØÄÜ“óĮæ¹²“ęµÄŹĒ
A. ŌŚŗ¬“óĮæFe3+µÄČÜŅŗÖŠ£ŗNH4+”¢Na+”¢Cl-”¢SCN-
B. ŌŚĒæ¼īČÜŅŗÖŠ£ŗNa+”¢K+”¢AlO2£”¢CO32£
C. c(H+)£½10-13 mol”¤LµÄČÜŅŗÖŠ£ŗNH4+”¢Al3+”¢SO42£”¢NO3£
D. ŌŚpH£½1µÄČÜŅŗÖŠ£ŗK+”¢Fe2+”¢Cl-”¢NO3£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķł100 mLäå»ÆŃĒĢśČÜŅŗÖŠ»ŗ»ŗĶØČė2.24 L(±ź×¼×“æöĻĀ)Cl2,·“Ó¦ĶźČ«ŗóČÜŅŗÖŠÓŠ1/3µÄäåĄė×Ó±»Ńõ»Æ³Éµ„ÖŹä唣ĒóŌäå»ÆŃĒĢśČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌĀČĄÖŠµÄ3HeŹĒŗĖ¾Ū±ä·¢µē×īĒå½ą×ī°²Č«µÄĄķĻėĪļÖŹ£¬µŲĒņÉĻŗ¤ŌŖĖŲÖ÷ŅŖŅŌ4HeŠĪŹ½“ęŌŚ£¬3He½öÓŠ15 t×óÓŅ£»¶ųŌĀĒņÉĻµÄ3HeÓŠŹż°ŁĶņ¶ÖÖ®¶ą£¬æɹ©Č«ŹĄ½ēæŖ²É500Äź”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
¢Ł3He”¢4HeµÄ»ÆѧŠŌÖŹ»ł±¾ĻąĶ¬””¢Ś3He”¢4He¾ßÓŠĻąĶ¬µÄÖŠ×ÓŹż””¢Ū3HeŗĖ¾Ū±äŹĒ»Æѧ±ä»Æ””¢Ü3HeŅŗ»ÆŹĒĪļĄķ±ä»Æ””¢Ż3He”¢4HeŠĪ³ÉµÄµ„ÖŹÖŠ¾łŗ¬ÓŠ¹²¼Ū¼ü””¢Ž3He”¢4He·Ö±š×é³ÉµÄĘųĢåµ„ÖŹ£¬ŌŚĻąĶ¬Ģõ¼žĻĀĆܶČÖ®±ČĪŖ3”Ć4
A£®¢Ł¢Ś¢Ż B£®¢Ł¢Ü¢Ž
C£®¢Ś¢Ū¢Ż D£®¢Ł¢Ū¢Ž
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼×Ķé·Ö×ÓŹĒŅŌĢ¼Ō×ÓĪŖÖŠŠÄµÄÕżĖÄĆęĢå½į¹¹¶ų²»ŹĒÕż·½ŠĪµÄĘ½Ćę½į¹¹£¬ĘäĄķÓÉŹĒ
(”””” )
A£®CHCl3Ö»ÓŠŅ»ÖÖ½į¹¹
B£®CH2Cl2Ö»ÓŠŅ»ÖÖ½į¹¹
C£®CH4ŗ¬ÓŠ¼«ŠŌ¼ü
D£®CH4µÄĖÄøö¼Ū¼üµÄ¼ü³¤ŗĶ¼üÄܶ¼ĻąµČ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com