
A.øĆ·“Ó¦ŹōÓŚ·Ö½ā·“Ó¦”¢Ńõ»Æ»¹Ō·“Ó¦
B.ÉĻŹö·“Ó¦Ė²¼äÄܲśÉś“óĮæøßĪĀĘųĢ壬ÕāŹĒĶĘ¶Æ·É“¬·ÉŠŠµÄÖ÷ŅŖŅņĖŲ
C.ĀĮ·ŪµÄ×÷ÓĆŹĒµćČ¼Ź±Ńõ»Æ·ÅČČŅż·¢øßĀČĖįļ§·“Ó¦
D.ŌŚ·“Ó¦ÖŠNH4ClO4½öĘšµ½Ńõ»Æ¼Į×÷ÓĆ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÖ¾ŗčĻµĮŠŃµĮ·±ŲŠŽŅ»»ÆѧČĖ½Ģ°ę ČĖ½Ģ°ę ĢāŠĶ£ŗ013
|
»š¼żµÄČ¼ĮĻ·ÖĪŖ¹ĢĢåČ¼ĮĻŗĶŅŗĢåČ¼ĮĻµČ£¬ŅŌĀĮ·ŪÓėøßĀČĖįļ§µÄ»ģŗĻĪļĪŖ¹ĢĢåČ¼ĮĻ£¬ĘäÖŠøßĀČĖįļ§µÄ·“Ó¦ĪŖ£ŗ2NH4ClO4 | |
| [””””] | |
A£® |
øĆ·“Ó¦ŹōÓŚ·Ö½ā·“Ó¦”¢Ńõ»Æ»¹Ō·“Ó¦ |
B£® |
ÉĻŹö·“Ó¦Ė²¼äÄܲśÉś“óĮæøßĪĀĘųĢ壬ÕāŹĒĶĘ¶Æ·É“¬·ÉŠŠµÄÖ÷ŅŖŅņĖŲ |
C£® |
ĀĮ·ŪµÄ×÷ÓĆŹĒµćČ¼Ź±Ńõ»Æ·ÅČČŅż·¢øßĀČĖįļ§·“Ó¦ |
D£® |
ŌŚ·“Ó¦ÖŠNH4ClO4½öĘšµ½Ńõ»Æ¼Į×÷ÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
(1)øł¾ŻŅŌÉĻŠÅĻ¢£¬ÅŠ¶ĻAgX”¢AgY”¢AgZČżÕßµÄČܽā¶Č(ÓĆŅѱ»ČܽāµÄČÜÖŹµÄĪļÖŹµÄĮæ/
(2)ČōĻņAgYµÄ±„ŗĶČÜŅŗÖŠ¼ÓČėÉŁĮæµÄAgX¹ĢĢ壬Ōņc(Y-)_______________(Ģī”°Ōö“ó”±”°¼õŠ””±»ņ”°²»±ä”±)”£
(3)ŌŚ
(4)ÓÉÉĻŹöKspÅŠ¶Ļ£¬ŌŚÉĻŹö(3)µÄĢåĻµÖŠ£¬___________(Ģī”°ÄÜ”±»ņ”°·ń”±)ŹµĻÖAgYĻņAgZµÄ×Ŗ»Æ£¬¼ņŹöĄķÓÉ£ŗ_______________________________________________________”£
(¢ņ) ”°ęĻ¶šŅ»ŗÅ”±³É¹¦·¢É䣬ŹµĻÖĮĖÖŠ¹śČĖµÄ”°±¼ŌĀ”±ĆĪĻė”£
(1)·¢Éä”°ęĻ¶šŅ»ŗÅ”±µÄ³¤Õ÷Čżŗż׻š¼żµÄµŚČż¼¶Ź¹ÓƵÄĶĘ½ų¼ĮŹĒŅŗĒāŗĶŅŗŃõ£¬ĻĀĮŠŹĒ298 KŹ±£¬ĒāĘų(H2)”¢Ģ¼(C)”¢ŠĮĶé(C8H18)”¢¼×Ķé(CH4)Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½£ŗH2(g)+![]() O2(g)
O2(g)![]() H2O(l)£»¦¤H=-285.8 kJ”¤mol
H2O(l)£»¦¤H=-285.8 kJ”¤mol![]() CO2(g)£»¦¤H=-393.5 kJ”¤mol
CO2(g)£»¦¤H=-393.5 kJ”¤mol![]() O2(g)
O2(g)![]() 8CO2(g)+9H2O(l)£»¦¤H=-5 518 kJ”¤mol-1 CH4(g)+2O2(g)
8CO2(g)+9H2O(l)£»¦¤H=-5 518 kJ”¤mol-1 CH4(g)+2O2(g)![]() CO2(g)+2H2O(l)£»¦¤H=-890.3 kJ”¤mol-1
CO2(g)+2H2O(l)£»¦¤H=-890.3 kJ”¤mol-1
Ķعż¼ĘĖćĖµĆ÷µČÖŹĮæµÄH2”¢C”¢C8H18”¢CH4ĶźČ«Č¼ÉÕŹ±£¬·Å³öČČĮæ×ī¶ąµÄŹĒ_____________£¬·¢Éä”°ęĻ¶šŅ»ŗÅ”±µÄ³¤Õ÷Čżŗż׻š¼żµÄµŚČż¼¶Ź¹ÓĆŅŗĒāŗĶŅŗŃõÕāÖÖĶĘ½ų¼ĮµÄÓŵćŹĒ__________
__________________________£»____________________________________”£(ĒėŠ“Į½Ģõ)
(2)ŅŃÖŖ£ŗH2(g)![]() H2(l)£»¦¤H=-0.92 kJ”¤mol-1
H2(l)£»¦¤H=-0.92 kJ”¤mol-1
O2(g)![]() O2(l)£»¦¤H=-6.84 kJ”¤mol-1
O2(l)£»¦¤H=-6.84 kJ”¤mol-1
H2O(l)![]() H2O(g)£»¦¤H=44.0 kJ”¤mol-1
H2O(g)£»¦¤H=44.0 kJ”¤mol-1
ĒėŠ“³öŅŗĒāŗĶŅŗŃõÉś³ÉĘųĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½______________________________________”£
Čē¹ū“Ė“Ī·¢Éä”°ęĻ¶šŅ»ŗÅ”±µÄ³¤Õ÷Čżŗż׻š¼żĖłŠÆ“ųµÄČ¼ĮĻĪŖ45¶Ö£¬ŅŗĒā”¢ŅŗŃõĒ”ŗĆĶźČ«·“Ӧɜ³ÉĘųĢ¬Ė®£¬×ܹ²ŹĶ·ÅÄÜĮæ________kJ(±£Įō3Ī»ÓŠŠ§Źż×Ö)”£
(3)ĒāĘų”¢ŃõĘų²»½öČ¼ÉÕŹ±ÄÜŹĶ·ÅČČÄÜ£¬¶žÕߊĪ³ÉµÄŌµē³Ų»¹ÄÜĢį¹©µēÄÜ£¬ĆĄ¹śµÄĢ½ŌĀ·É“¬”°°¢²ØĀŽŗÅ”±Ź¹ÓĆµÄ¾ĶŹĒĒāŃõČ¼ĮĻµē³Ų£¬µē½āŅŗĪŖKOHČÜŅŗ£¬Ęäµē³Ų·“Ó¦Ź½ĪŖ£ŗøŗ¼«£ŗ____________£»Õż¼«£ŗ____________£»×Ü·“Ó¦Ź½£ŗ________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗÓ±±Ź”øßČż3ŌĀĆžµ×æ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¼×“¼Č¼ĮĻ·ÖĪŖ¼×“¼ĘūÓĶŗĶ¼×“¼²ńÓĶ”£¹¤ŅµÉĻŗĻ³É¼×“¼µÄ·½·Øŗܶą”£
£Ø1£©Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗ
CO2(g) +3H2(g) £½CH3OH(g)+H2O(g)? ”÷H1
2CO (g) +O2(g) £½2CO2(g)?? ”÷H2
2H2(g)+O2(g) £½2H2O(g)??? ”÷H3
Ōņ””CO(g) + 2H2(g)  CH3OH(g)””µÄ”÷H£½??????????????? ”£
CH3OH(g)””µÄ”÷H£½??????????????? ”£
£Ø2£©ŌŚČŻ»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£ŗCO(g)+2H2(g) CH3OH(g) £¬ĘäĖūĢõ¼ž²»±ä£¬ŌŚ300”ęŗĶ500”ꏱ£¬ĪļÖŹµÄĮæn(CH3OH) Óė·“Ó¦Ź±¼ätµÄ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£øĆ·“Ó¦µÄ”÷H???? 0 £ØĢī>”¢<»ņ=£©”£
CH3OH(g) £¬ĘäĖūĢõ¼ž²»±ä£¬ŌŚ300”ęŗĶ500”ꏱ£¬ĪļÖŹµÄĮæn(CH3OH) Óė·“Ó¦Ź±¼ätµÄ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£øĆ·“Ó¦µÄ”÷H???? 0 £ØĢī>”¢<»ņ=£©”£

£Ø3£©ČōŅŖĢįø߼ד¼µÄ²śĀŹ£¬æɲÉČ”µÄ“ėŹ©ÓŠ____________£ØĢī×ÖÄø£©”£
A£®ĖõŠ”ČŻĘ÷Ģå»ż
B£®½µµĶĪĀ¶Č
C£®ÉżøßĪĀ¶Č
D£®Ź¹ÓĆŗĻŹŹµÄ“߻ƼĮ
E£®½«¼×“¼“Ó»ģŗĻĢåĻµÖŠ·ÖĄė³öĄ“
£Ø4£©CH4ŗĶH2OŌŚ“߻ƼĮ±ķĆę·¢Éś·“Ó¦CH4+H2O CO+3H2£¬T”ꏱ£¬Ļņ1 LĆܱÕČŻĘ÷ÖŠĶ¶Čė1 mol CH4ŗĶ1 mol H2O(g)£¬5Š”Ź±ŗó²āµĆ·“Ó¦ĢåĻµ“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±CH4µÄ×Ŗ»ÆĀŹĪŖ50% £¬¼ĘĖćøĆĪĀ¶ČĻĀµÄĘ½ŗā³£Źż????? £Ø½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£©”£
CO+3H2£¬T”ꏱ£¬Ļņ1 LĆܱÕČŻĘ÷ÖŠĶ¶Čė1 mol CH4ŗĶ1 mol H2O(g)£¬5Š”Ź±ŗó²āµĆ·“Ó¦ĢåĻµ“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±CH4µÄ×Ŗ»ÆĀŹĪŖ50% £¬¼ĘĖćøĆĪĀ¶ČĻĀµÄĘ½ŗā³£Źż????? £Ø½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£©”£
£Ø5£©ŅŌ¼×“¼ĪŖČ¼ĮĻµÄŠĀŠĶµē³Ų£¬Ęä³É±¾“ó“óµĶÓŚŅŌĒāĪŖČ¼ĮĻµÄ “«Ķ³Č¼ĮĻµē³Ų£¬ÄæĒ°µĆµ½¹ć·ŗµÄŃŠ¾æ£¬ČēĶ¼ŹĒÄæĒ°ŃŠ¾æ½Ļ¶ąµÄŅ»Ąą¹ĢĢåŃõ»ÆĪļČ¼ĮĻµē³Ų¹¤×÷ŌĄķŹ¾ŅāĶ¼”£»Ų“šĻĀĮŠĪŹĢā£ŗ
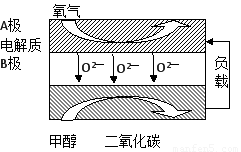
¢ŁB¼«µÄµē¼«·“Ó¦Ź½ĪŖ????????????????????? ”£
¢ŚČōÓĆøĆČ¼ĮĻµē³Ų×öµēŌ“£¬ÓĆŹÆÄ«×öµē¼«µē½āĮņĖįĶČÜŅŗ£¬µ±µēĀ·ÖŠ×ŖŅĘ1mole- Ź±£¬Źµ¼ŹÉĻĻūŗĵļד¼µÄÖŹĮæ±ČĄķĀŪÉĻ“ó£¬æÉÄÜŌŅņŹĒ????????????????????? ”£
£Ø6£©25”ꏱ£¬²ŻĖįøʵÄKsp=4.0”Į10-8,Ģ¼ĖįøʵÄKsp=2.5”Į10-9”£Ļņ20mlĢ¼Ėįøʵı„ŗĶČÜŅŗÖŠÖšµĪ¼ÓČė8.0”Į10-4 mol”¤L-1µÄ²ŻĖį¼ŲČÜŅŗ20ml£¬ÄÜ·ń²śÉś³Įµķ?????? £ØĢī”°ÄÜ”±»ņ”°·ń”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014Ń§ÄźÉ½Ī÷Ź”ĖÄŠ£øßČżµŚĖÄ“ĪĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¼×“¼Č¼ĮĻ·ÖĪŖ¼×“¼ĘūÓĶŗĶ¼×“¼²ńÓĶ”£¹¤ŅµÉĻŗĻ³É¼×“¼µÄ·½·Øŗܶą”£
£Ø1£©Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗ
¢ŁCO2£Øg£© +3H2£Øg£© £½CH3OH£Øg£©+H2O£Øg£© ? ”÷H1????
¢Ś2CO£Øg£© +O2£Øg£© £½2CO2£Øg£©?? ”÷H2
¢Ū2H2£Øg£©+O2£Øg£© £½2H2O£Øg£©????????? ”÷H3
ŌņCO£Øg£© + 2H2£Øg£©  CH3OH£Øg£©””µÄ”÷H£½??????????????? ”£
CH3OH£Øg£©””µÄ”÷H£½??????????????? ”£
£Ø2£©ŌŚČŻ»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£ŗ CO£Øg£©+2H2£Øg£© CH3OH£Øg£© £¬ĘäĖūĢõ¼ž²»±ä£¬ŌŚ300”ęŗĶ500”ꏱ£¬ĪļÖŹµÄĮæn£ØCH3OH£© Óė·“Ó¦Ź±¼ätµÄ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£øĆ·“Ó¦µÄ”÷H???? 0 £ØĢī>”¢<»ņ=£©”£
CH3OH£Øg£© £¬ĘäĖūĢõ¼ž²»±ä£¬ŌŚ300”ęŗĶ500”ꏱ£¬ĪļÖŹµÄĮæn£ØCH3OH£© Óė·“Ó¦Ź±¼ätµÄ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£øĆ·“Ó¦µÄ”÷H???? 0 £ØĢī>”¢<»ņ=£©”£

£Ø3£©ČōŅŖĢįø߼ד¼µÄ²śĀŹ£¬æɲÉČ”µÄ“ėŹ©ÓŠ____________£ØĢī×ÖÄø£©”£
A£®ĖõŠ”ČŻĘ÷Ģå»ż
B£®½µµĶĪĀ¶Č
C£®ÉżøßĪĀ¶Č
D£®Ź¹ÓĆŗĻŹŹµÄ“߻ƼĮ
E£®½«¼×“¼“Ó»ģŗĻĢåĻµÖŠ·ÖĄė³öĄ“
£Ø4£©CH4ŗĶH2OŌŚ“߻ƼĮ±ķĆę·¢Éś·“Ó¦CH4+H2O CO+3H2£¬T”ꏱ£¬Ļņ1 LĆܱÕČŻĘ÷ÖŠĶ¶Čė1 mol CH4ŗĶ1 mol H2O£Øg£©£¬5Š”Ź±ŗó²āµĆ·“Ó¦ĢåĻµ“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±CH4µÄ×Ŗ»ÆĀŹĪŖ50% £¬¼ĘĖćøĆĪĀ¶ČĻĀµÄĘ½ŗā³£Źż???? £Ø½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£©”£
CO+3H2£¬T”ꏱ£¬Ļņ1 LĆܱÕČŻĘ÷ÖŠĶ¶Čė1 mol CH4ŗĶ1 mol H2O£Øg£©£¬5Š”Ź±ŗó²āµĆ·“Ó¦ĢåĻµ“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±CH4µÄ×Ŗ»ÆĀŹĪŖ50% £¬¼ĘĖćøĆĪĀ¶ČĻĀµÄĘ½ŗā³£Źż???? £Ø½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£©”£
£Ø5£©ŅŌ¼×“¼ĪŖČ¼ĮĻµÄŠĀŠĶµē³Ų£¬Ęä³É±¾“ó“óµĶÓŚŅŌĒāĪŖČ¼ĮĻµÄ“«Ķ³Č¼ĮĻµē³Ų£¬ÄæĒ°µĆµ½¹ć·ŗµÄŃŠ¾æ£¬ČēĶ¼ŹĒÄæĒ°ŃŠ¾æ½Ļ¶ąµÄŅ»Ąą¹ĢĢåŃõ»ÆĪļČ¼ĮĻµē³Ų¹¤×÷ŌĄķŹ¾ŅāĶ¼”£»Ų“šĻĀĮŠĪŹĢā£ŗ

¢ŁB¼«µÄµē¼«·“Ó¦Ź½ĪŖ????????????????????? ”£
¢ŚČōÓĆøĆČ¼ĮĻµē³Ų×öµēŌ“£¬ÓĆŹÆÄ«×öµē¼«µē½āĮņĖįĶČÜŅŗ£¬µ±µēĀ·ÖŠ×ŖŅĘ1mole- Ź±£¬Źµ¼ŹÉĻĻūŗĵļד¼µÄÖŹĮæ±ČĄķĀŪÉĻ“ó£¬æÉÄÜŌŅņŹĒ????????????????????? ”£
£Ø6£©25”ꏱ£¬²ŻĖįøʵÄKsp=4.0”Į10-8,Ģ¼ĖįøʵÄKsp=2.5”Į10-9”£Ļņ20mlĢ¼Ėįøʵı„ŗĶČÜŅŗÖŠÖšµĪ¼ÓČė8.0”Į10-4 mol”¤L-1µÄ²ŻĖį¼ŲČÜŅŗ20ml£¬ÄÜ·ń²śÉś³Įµķ?????? £ØĢī”°ÄÜ”±»ņ”°·ń”±£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com