
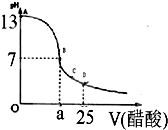
 ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ���ᣬ��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ���ᣬ��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
| c(��)V(��) |
| V(��) |
| c(��)V(��) |
| V(��) |
| 23.00+23.02 |
| 2 |
| 0.1mol/L��0.02301L |
| 0.020L |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ����,��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����=�� )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�������Ũ��ԼΪ___________________(������λ��Ч����)��
�ζ��ﵽ�յ�ı�־��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ȹ㶫ʡտ�����и߶���һѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣�ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ����,��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����="��" )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȹ㶫ʡ�߶���һѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣�ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ����,��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
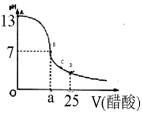
��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����=�� )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
|
ʵ����� |
��һ�� |
�ڶ��� |
������ |
|
����NaOH��Һ���/mL |
19.00 |
23.00 |
23.02 |
�������Ũ��ԼΪ___________________ (������λ��Ч����)��
�ζ��ﵽ�յ�ı�־��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡտ�����и߶����ϣ���ĩ��ѧ�Ծ������ƣ��������棩 ���ͣ������
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
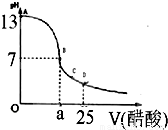
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com