������CH
2=CH-CH�TCH
2�ķ���ʽΪC
4H
6�������������ķ�Ӧ�У���C
4H
6+H
2��C
4H
8��C
4H
6+H
2��C
4H
10��C
4H
6+2H
2��C
4H
10��
��V��C
4H
6����V��H
2����1ʱ����Ӧ�����բٽ��У���V��C
4H
6����V��H
2����
ʱ����Ӧ���۽��У���
��V��C
4H
6����V��H
2����1ʱ�����ְ��ٽ��У����ְ��۷�Ӧ��
��V��C
4H
6����V��H
2����
ʱ�����۷����ٻ�ۣ��õ��IJ�����C
4H
6����C
4H
10�����ǵĻ���ʣ�������������ڷ�Ӧ��C
4H
6�������
��V��C
4H
6����V��H
2����
ʱ������������ʣ������Ϊ��C
4H
6��H
2�Ļ����������ΪC
4H
6��ʣ��H
2�Ļ���
��1�������������Ϊ1L����Ӧ������Ϊ1��3-����ϩ�������
��2����V��C
4H
6����V��H
2����
ʱ����x��
���õ��IJ�����C
4H
6����C
4H
10�����ǵĻ���ʣ�������������ڷ�Ӧ��C
4H
6�������
��V��C
4H
6����V��H
2����
ʱ����x��
������������ʣ��������������ΪC
4H
6��ʣ��H
2�Ļ���
��3����x=
ʱ�������������ﵽ��Сֵ��x��
������x�����ӣ�����������V������x��
������x�����������V��С���ݴ˻���xΪ��ֵͬʱ��Ӧ������V��ͼ��
��4����i����Ȳ��1��3-����ϩ�������ķ�Ӧ��ϵ��ͬ����y=0.2ʱ���������ĺ���Ϊ��1-0.2=0.8���������ĺ���x�ķ�ΧΪ��0��x��0.8�������������V���������x��ͼ���루3�����ƣ�������ʱx�ķ�ΧΪ0��x��0.8���ݴ˻�����y=0.2ʱ��V��x�仯�����ߣ�
��ii�������Һ���Ϊyʱ�������ĺ���xΪ��0��x��1-y��
��x=0.15ʱ����Ȳ��1��3-����ϩ���ܺ���Ϊ��1-0.15=0.85��
����x=0.15��
�����ܷ���ʲô��Ӧ������������������Ӧ����������Ϊ��Ȳ��1��3-����ϩ�����֮�ͣ�Ϊ��5L��0.85=4.25L���ݴ˻���x=0.15ʱ��V��y�仯�����ߣ�
����⣺CH
2=CH-CH�TCH
2�ķ���ʽΪC
4H
6�������������ķ�Ӧ�У���C
4H
6+H
2��C
4H
8��C
4H
6+H
2��C
4H
10��C
4H
6+2H
2��C
4H
10��
��V��C
4H
6����V��H
2����1ʱ����Ӧ�����բٽ��У�
��V��C
4H
6����V��H
2����
ʱ����Ӧ���۽��У�
��
��V��C
4H
6����V��H
2����1ʱ�����ְ��ٽ��У����ְ��۷�Ӧ��
��V��C
4H
6����V��H
2����
ʱ�����۷����ٻ�ۣ��õ��IJ�����C
4H
6����C
4H
10�����ǵĻ���ʣ�������������ڷ�Ӧ��C
4H
6�������
��V��C
4H
6����V��H
2����
ʱ������������ʣ������Ϊ��C
4H
6��H
2�Ļ����������ΪC
4H
6��ʣ��H
2�Ļ���
��1�������������Ϊ1L����1��3-����ϩ�����Ϊ��5L-1L=4L����Ӧ������Ϊ1��3-����ϩ�������4L��
�𣺷�Ӧ������VΪ4L��
��2����V��C
4H
6����V��H
2����
ʱ����x��
���õ��IJ�����C
4H
6����C
4H
10�����ǵĻ���ʣ�������������ڷ�Ӧ��C
4H
6�������V=V��C
4H
6��=5��1-x��L��
��V��C
4H
6����V��H
2����
ʱ����x��
������������ʣ��������������ΪC
4H
6��ʣ��H
2�Ļ���V=V��C
4H
6��+V
ʣ����H
2��=5��1-x��L+5x-2��5��1-x��=10x-5��
��xΪ��ֵͬʱ��Ӧ������VΪ10x-5��
��3����x=
ʱ�������������ﵽ��Сֵ��x��
������x�����ӣ�����������V������x��
������x�����������V��С���ݴ˻���xΪ��ֵͬʱ��Ӧ������V��ͼ��Ϊ��

��
��xΪ��ֵͬʱ��Ӧ������V��ͼ��Ϊ

��
��4����i����Ȳ��1��3-����ϩ�������ķ�Ӧ��ϵ��ͬ����y=0.2ʱ���������ĺ���Ϊ��1-0.2=0.8���������ĺ���x�ķ�ΧΪ��0��x��0.8�������������V���������x��ͼ���루3�����ƣ�������ʱx�ķ�ΧΪ0��x��0.8���ݴ˻�����y=0.2ʱ��V��x�仯������Ϊ��

��
�𣺵�y=0.2ʱ��V��x�仯������Ϊ

��
��ii�������Һ���Ϊyʱ�������ĺ���xΪ��0��x��1-y��
��x=0.15ʱ����Ȳ��1��3-����ϩ���ܺ���Ϊ��1-0.15=0.85��
����x=0.15��
�����ܷ���ʲô��Ӧ������������������Ӧ����������Ϊ��Ȳ��1��3-����ϩ�����֮�ͣ�Ϊ��5L��0.85=4.25L����x=0.15ʱ��V��y�仯������Ϊ��

����x=0.15ʱ��V��y�仯������Ϊ

��

 ��1��3-����ϩ�������Ļ������5L���ڴ��������£�����ַ�Ӧ��õ����������ΪVL�������������ͬ�¡�ͬѹ�²ⶨ����
��1��3-����ϩ�������Ļ������5L���ڴ��������£�����ַ�Ӧ��õ����������ΪVL�������������ͬ�¡�ͬѹ�²ⶨ���� ��
�� ��
�� ��
�� ��
�� ����x=0.15ʱ��V��y�仯������Ϊ
����x=0.15ʱ��V��y�仯������Ϊ ��
��

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�

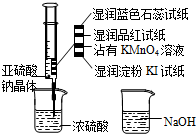 ��ͼ����ע�����м�������Na2SO3���壬����������Ũ���ᣨ�Բ��Ӵ�ֽ��Ϊ�����������й�˵����ȷ���ǣ�������
��ͼ����ע�����м�������Na2SO3���壬����������Ũ���ᣨ�Բ��Ӵ�ֽ��Ϊ�����������й�˵����ȷ���ǣ�������