
£Ø17·Ö£©¼×”¢ŅŅ”¢±ūŹĒ֊ѧ֊³£¼ūµÄµ„ÖŹ£¬X”¢Y”¢ZŹĒ³£¼ūµÄ»ÆŗĻĪļ”£ŌŚ³£ĪĀ³£Ń¹ĻĀ£¬¼×ŹĒ¾ßÓŠŃõ»ÆŠŌµÄ»ĘĀĢÉ«ĘųĢ壬±ūŹĒ×ŲŗģÉ«µÄŅŗĢ壬YÓėZŗ¬ÓŠĻąĶ¬µÄŃōĄė×Ó£¬XÓėZŗ¬ÓŠĻąĶ¬µÄŅõĄė×Ó£»ĖüĆĒÖ®¼äÓŠŅŌĻĀ×Ŗ»Æ¹ŲĻµ£ŗ±ūŹ®ŅŅ
”śZ;XŹ®±ū”śZ£»X+¼×”śY+±ū”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³ö¼×”¢ŅŅ”¢±ūČżÖÖĪļÖŹµÄ»ÆѧŹ½___ _”¢ ”¢___ _£»
£Ø2£©Š“³öXÓė×ćĮæµÄ¼×ŌŚČÜŅŗÖŠĶźČ«·“Ó¦Ź±µÄĄė×Ó·½³ĢŹ½ ”£
£Ø3£©ÓūŌŚŹµŃéŹŅÖŠÖĘČ”²¢ŹÕ¼Æ“æ¾»”¢øÉŌļµÄĘųĢå¼×£¬Č»ŗóĶź³ÉÉĻŹö·“Ó¦”°X+¼×”śY+±ū”±£¬Ä³Ķ¬Ń§Éč¼ĘĮĖČēĻĀĶ¼ĖłŹ¾µÄ×°ÖĆ”£
¢Ł×°ÖĆAÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
¢Ś×°ÖĆBµÄ×÷ÓĆŹĒ £»
¢Ū×°ÖĆCÖŠµÄŹŌ¼ĮĪŖ £»
¢Ü×°ÖĆDÖŠŹÕ¼ÆĘųĢå¼×µÄ·½·ØĆū³ĘŹĒ £»
¢Ż×°ÖĆFµÄÖ÷ŅŖ×÷ÓĆŹĒ £¬·“Ó¦ŌĄķµÄĄė×Ó·½³ĢŹ½ĪŖ ”£
 ±¦±“¼Ę»®ĘŚÄ©³å“Ģ¶į100·ÖĻµĮŠ“š°ø
±¦±“¼Ę»®ĘŚÄ©³å“Ģ¶į100·ÖĻµĮŠ“š°ø ÄÜæ¼ŹŌČ«ÄÜ100·ÖĻµĮŠ“š°ø
ÄÜæ¼ŹŌČ«ÄÜ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĪļĄķ½ĢŃŠŹŅ ĢāŠĶ£ŗ022
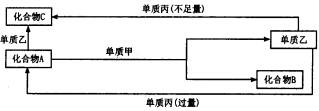
ŹŌ»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©Čō¼××÷»¹Ō¼Į£¬¼×ŹĒ³£¼ū½šŹōµ„ÖŹ£¬ŅŅŹĒ³£¼ū·Ē½šŹōµ„ÖŹ£¬ŌņCµÄ»ÆѧŹ½ŹĒ ________£¬AµÄµē×ÓŹ½ŹĒ________£¬AÓė¼×·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________”£
£Ø2£©Čō¼××÷Ńõ»Æ¼Į£¬¼×ŹĒ³£¼ūµÄ·Ē½šŹōµ„ÖŹ£¬±ūŹĒ³£¼ū½šŹō£¬ĒŅ·“Ó¦¶¼ŹĒŌŚČÜŅŗÖŠĶØ ³£Ģõ¼žĻĀ½ųŠŠ£¬Ōņ£ŗ¢ŁCµÄ»ÆѧŹ½ŹĒ________£¬¢Ś»ÆŗĻĪļAÓėµ„ÖŹŅŅ·“Ó¦µÄøß×Ó·½³Ģ Ź½ŹĒ________”£»ÆŗĻĪļCÓėµ„ÖŹ±ū·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ________”£»ÆŗĻĪļAÓėµ„ÖŹ¼× µÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ3Ź±£¬AÓė¼×Ē”ŗĆĶźČ«·“Ó¦ĒŅ·ūŗĻÉĻĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£¬Ōņ·“Ó¦ µÄĄė×Ó·½³ĢŹ½ŹĒ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ043
A£¬B£¬CŹĒ֊ѧ»Æѧ֊³£¼ūµÄĪŽ»śĪļ£¬ĒŅø÷ÓÉĮ½ÖÖŌŖĖŲ×é³É£¬¼×”¢ŅŅ”¢±ūŹĒ ČżÖÖ³£¼ūµÄµ„ÖŹ£¬ÕāŠ©»ÆŗĻĪļŗĶµ„ÖŹÖ®¼ä“ęŌŚČēĻĀĶ¼ĖłŹ¾×Ŗ»Æ¹ŲĻµ£ØÕāŠ©×Ŗ»Æ¶¼²»ŠčŅŖŹ¹ÓĆ“ß»Æ¼Į£©”£
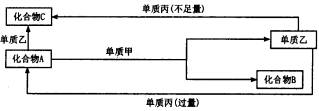
ŹŌ»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©Čō¼××÷»¹Ō¼Į£¬¼×ŹĒ³£¼ū½šŹōµ„ÖŹ£¬ŅŅŹĒ³£¼ū·Ē½šŹōµ„ÖŹ£¬ŌņCµÄ»ÆѧŹ½ŹĒ ________£¬AµÄµē×ÓŹ½ŹĒ________£¬AÓė¼×·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________”£
£Ø2£©Čō¼××÷Ńõ»Æ¼Į£¬¼×ŹĒ³£¼ūµÄ·Ē½šŹōµ„ÖŹ£¬±ūŹĒ³£¼ū½šŹō£¬ĒŅ·“Ó¦¶¼ŹĒŌŚČÜŅŗÖŠĶØ ³£Ģõ¼žĻĀ½ųŠŠ£¬Ōņ£ŗ¢ŁCµÄ»ÆѧŹ½ŹĒ________£¬¢Ś»ÆŗĻĪļAÓėµ„ÖŹŅŅ·“Ó¦µÄøß×Ó·½³Ģ Ź½ŹĒ________”£»ÆŗĻĪļCÓėµ„ÖŹ±ū·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ________”£»ÆŗĻĪļAÓėµ„ÖŹ¼× µÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ3Ź±£¬AÓė¼×Ē”ŗĆĶźČ«·“Ó¦ĒŅ·ūŗĻÉĻĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£¬Ōņ·“Ó¦ µÄĄė×Ó·½³ĢŹ½ŹĒ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖA”¢BĪŖ³£¼ūµÄ½šŹōµ„ÖŹ£¬C”¢DĪŖ³£¼ūµÄ·Ē½šŹōµ„ÖŹ£¬¼×”¢ŅŅ”¢±ūĪŖČżÖÖ³£¼ūµÄ»ÆŗĻĪļ£¬ĖüĆĒÖ®¼äµÄĻą»„×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö²śĪļ¼°·“Ó¦Ģõ¼žĆ»ÓŠĮŠ³ö£©£ŗ

£Ø1£©Čō¼×ŹĒ֊ѧ»Æѧ֊³£¼ūµÄŗģ×ŲÉ«·Ūĩד¹ĢĢ壬Ōņµ„ÖŹAÓėNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»BŌŚ³±ŹŖµÄæÕĘųÖŠŅ×·¢Éśµē»ÆѧøÆŹ“£¬Š“³öĘä·¢Éśµē»ÆѧøÆŹ“Ź±Ōµē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½£ŗ ”£
£Ø2£©ČōŅŅŌŚĖ®ČÜŅŗ³ŹČõĖįŠŌ£¬±ūŹĒŅ»ÖÖ“óĘųĪŪČ¾Īļ£¬ÓŠ“Ģ¼¤ŠŌĘųĪ¶”£ŹŌŠ“³öŅŅŌŚĖ®ČÜŅŗÖŠ·¢ÉśµēĄėŹ±µÄµēĄė·½³ĢŹ½ £»ŅŃÖŖ16g¹ĢĢåµ„ÖŹDĶźČ«Č¼ÉÕ×Ŗ»Æ³ÉŅŅŹ±£¬·Å³ö148.4kJµÄČČĮ棬ŌņøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©ČōŅŅŌŚĖ®ČÜŅŗ³ŹČõ¼īŠŌ£¬²¢æÉÓĆ×÷Å©Ņµ»Æ·Ź£¬D³£æöĻĀĪŖĘųĢ壬Ōņ½ųŠŠĻĀĮŠŃŠ¾æ£ŗ ĻÖ½«0.40 mol CŗĶ0.20 mol D³äČė10LµÄĆܱÕČŻĘ÷ÖŠ£¬ŌŚŅ»¶ØĢõ¼žĻĀŹ¹Ęä·¢Éś·“Ó¦£¬ÓŠ¹ŲC”¢D”¢ŅŅČżÕßµÄĪļÖŹµÄĮæµÄ±ä»ÆÓėŹ±¼äµÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾£ŗ
¢ŁČōt1 = 10min£¬Ōņ0ÖĮt1Ź±¼äÄŚCĪļÖŹµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ £»øĆ·“Ó¦ŌŚt2Ź±“ļµ½Ę½ŗā£¬Ęä»Æѧ·“Ó¦·½³ĢŹ½ĪŖ £¬“ĖĪĀ¶ČĻĀµÄøĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ ”£
¢Śøł¾ŻĶ¼ÖŠĒśĻß±ä»ÆĒéæöÅŠ¶Ļ£¬t1Ź±æĢøıäµÄ·“Ó¦Ģõ¼žæÉÄÜŹĒ £ØĢīĻĀĮŠø÷ĻīŠņŗÅ£©
a£®¼ÓČėĮĖ“߻ƼĮ
b£®½µµĶĮĖ·“Ó¦µÄĪĀ¶Č
c£®ĻņČŻĘ÷ÖŠÓÖ³äČėĮĖĘųĢåD

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com