
ĆŗŹĒÖŲŅŖµÄÄÜŌ“£¬Ņ²ŹĒ»Æ¹¤Éś²śµÄÖŲŅŖŌĮĻ”£
(1)ĆŗČ¼ÉÕ²śÉśµÄ·ĻĘųÖ±½ÓÅŷŵ½æÕĘųÖŠ£¬æÉÄܵ¼ÖĀµÄ»·¾³ĪŪČ¾ĪŹĢāŹĒ_______”£
(2)ĻĀĶ¼ŹĒ¶ŌĆŗæóČ¼ÉÕ²śÉśµÄ·ĻĘų½ųŠŠ³£ĪĀĶŃĮņ“¦ĄķµÄĮ÷³ĢŹ¾ŅāĶ¼”£
¢Ł·ĻĘųĶŃĮņ¹ż³ĢÖŠ£¬Ö÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________”¢_____________£»
¢ŚŌŚĆŗæóÖŠÖ±½ÓĢķ¼ÓŅ»ÖÖĪļÖŹ£¬æÉÓŠŠ§¼õÉŁĆŗæóČ¼ÉÕ²śÉśµÄSO2£¬øĆĪļÖŹŹĒ___£»
¢ŪŹÆøąµÄ¹¤ŅµÉś²śÖŠµÄÓĆĶ¾ŹĒ_________________(Š“³öŅ»ÖÖÓĆĶ¾¼“æÉ)”£
(3)Ćŗ¾¹ż___________(Ģī¼Ó¹¤·½·Ø)æÉŅŌµĆµ½½¹ĀÆĆŗĘų”¢Ćŗæó½¹ÓĶŗĶ½¹Ģ攣Ćŗ½¹ÓĶ¾¹ż_______(Ģī¼Ó¹¤·½·Ø)æɵƵ½·¼µĆ×å»ÆŗĻĪļ”£ĆŗæóŅ²æÉŅŌÓĆĒā»Æ·Ø×Ŗ»ÆČ¼ÓĶ£¬Ēā»Æ·ØµÄ±¾ÖŹŹĒ______________”£
(4)ĆŗæÉŅŌŅŗ»Æ×Ŗ»ÆĪŖCH3OH£¬ÓŠČĖÉč¼Ę³öŌŚKOHČÜŅŗÖŠÓƵē¼«±ķĆę¶ĘŅ»²ćĻøŠ”µÄ²¬·Ū×öµē¼«£¬²¬Īüø½ĘųĢåµÄÄÜĮ¦Ē棬ŠŌÖŹĪČ¶Ø£¬ĄūÓĆCH3OHŗĶO2¹¹³ÉµÄČ¼ĮĻµē³ŲµÄÄÜĮæ×Ŗ»ÆÖ÷ŅŖŠĪŹ½ŹĒ”””” £¬øŗ¼«·“Ó¦Ź½ĪŖ”””””””” £¬µē¼«±ķĆę¶Ę²¬·ŪµÄŌŅņĪŖ ”””””£
(1)ĖįÓź”¢·Ū³¾ĪŪČ¾
(2)¢ŁSO2£«CaCO3 CaSO3£«CO2”¢2CaSO3£«O2£½2CaSO4
¢ŚCaCO3”¢CaO”¢Ca(OH)2
¢ŪŅ½ÓĆ”¢½ØÖž²ÄĮĻ”¢Ä£ŠĶµČŗĻĄķ“š°ø
(3)øÉĮó£»·ÖĮó£»Ōö¼ÓĆŗÖŠĒāŌŖĖŲµĆŗ¬Į棬ĢįøßĒāŌ×ÓŹżÓėĢ¼Ō×ÓŹżµÄ±ČÖµ
(4)ÓÉ»ÆѧÄÜ×Ŗ»ÆĪŖµēÄÜ£»CH3OH+8OH- -6e-=CO32-+6H2O£»Ōö“óµē¼«µ„Ī»Ć껿Īüø½CH3OH”¢O2·Ö×ÓŹż£¬¼ÓĖŁµē¼«·“Ó¦ĖŁĀŹ”£
½āĪö


 ³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŌŖĖŲ¢ŁCl”¢¢ŚNa”¢¢ŪBr”¢¢ÜI”¢¢ŻMg”¢¢ŽUŹōÓŚŗ£Ė®ÖŠµÄĪ¢ĮæŌŖĖŲµÄŹĒ
| A£®¢Ł¢Ś¢Ż | B£®¢Ü¢Ž | C£®¢Ł¢Ś¢Ū¢Ü | D£®¢Ū¢Ü¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
“Óŗ£Ė®ÖŠæÉŅŌ»ńµĆµĖ®”¢Ź³ŃĪ£¬²¢æÉĢįČ”Ć¾ŗĶäåµČĪļÖŹ”£
(1)ŗ£Ė®µ»ÆµÄ·½·ØÖ÷ŅŖÓŠ________(ĢīŅ»ÖÖ)”£
(2)“Óŗ£Ė®ÖŠĢįČ”Ć¾µÄĮ÷³ĢČēĻĀĶ¼ĖłŹ¾£ŗ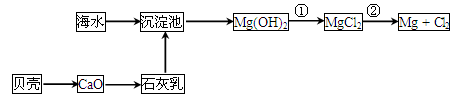
·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ________________________________________________”£
·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________________”£
(3)“Óŗ£Ė®ÖŠĢįČ”äåµÄÖ÷ŅŖ²½ÖčŹĒĻņÅØĖõµÄŗ£Ė®ÖŠĶØČėĀČĘų£¬½«äåĄė×ÓŃõ»Æ£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖ“śÉē»įÖŠĶŌŚµēĘų”¢½»ĶØ”¢»śŠµŗĶŅ±½š”¢ÄÜŌ“¼°ŹÆ»Æ¹¤Ņµ”¢øßæĘ¼¼µČĮģÓņÓŠ¹ć·ŗµÄÓ¦ÓĆ”£Ä³ĶæóŹÆŗ¬Ńõ»ÆĶ”¢Ńõ»ÆŃĒĶ”¢ČżŃõ»Æ¶žĢśŗĶĀöŹÆ(SiO2)£¬ĻÖ²ÉÓĆĖį½ž·Ø“ÓæóŹÆÖŠĢįČ”Ķ£¬Ę乤ŅÕĮ÷³ĢĶ¼ČēĻĀ”£ĘäÖŠĶµÄŻĶČ”(Ķ“ÓĖ®²ć½ųČėÓŠ»ś²ćµÄ¹ż³Ģ)ŗĶ·“ŻĶČ”(Ķ“ÓÓŠ»ś²ć½ųČėĖ®²ćµÄ¹ż³Ģ)ŹĒĻÖ“śŹŖ·ØĮ¶ĶµÄÖŲŅŖ¹¤ŅÕŹÖ¶Ī”£
ŅŃÖŖ£ŗ¢ŁCu£«ŌŚĖįŠŌČÜŅŗÖŠ²»ĪČ¶Ø£¬æÉ·¢Éś×ŌÉķŃõ»Æ»¹Ō·“Ó¦£»¢Śµ±æóŹÆÖŠČżŃõ»Æ¶žĢśŗ¬ĮæĢ«µĶŹ±£¬æÉÓĆĮņĖįŗĶĮņĖįĢśµÄ»ģŗĻŅŗ½ž³öĶ£»¢Ū·“ŻĶČ”ŗóµÄĖ®²ć2ŹĒĮņĖįĶČÜŅŗ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)æóŹÆÓĆĻ”ĮņĖį“¦Ąķ¹ż³ĢÖŠCu2O·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
(2)”°Ń»·¢ń”±¾¶ą“ĪŃ»·ŗóµÄĖ®²ć1²»ÄܼĢŠųŃ»·Ź¹ÓĆ£¬µ«æÉ·ÖĄė³öŅ»ÖÖÖŲŅŖµÄĮņĖįŃĪ¾§Ģ壬øĆ¾§ĢåµÄ»ÆѧŹ½ŹĒ ”£ČōĖ®²ć1±©Ā¶ŌŚæÕĘųÖŠŅ»¶ĪŹ±¼äŗó£¬æÉŅŌµĆµ½ĮķŅ»ÖÖÖŲŅŖµÄĮņĖįŃĪ£¬Š“³öĖ®²ć1±©Ā¶ŌŚæÕĘųÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
(3)Š“³öµē½ā¹ż³ĢÖŠŃō¼«(¶čŠŌµē¼«)·¢Éś·“Ó¦µÄµē¼«·“Ó¦Ź½£ŗ ”£
(4)”°Ń»·¢ó”±ÖŠ·“ŻĶČ”¼ĮµÄÖ÷ŅŖ³É·ÖŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŗ£ŃóŹĒøö¾Ž“óµÄ׏Ō“±¦æā£¬ŗ£Ė®×ŹŌ“µÄĄūÓĆ¾ßÓŠ·Ē³£¹ćĄ«µÄ·¢Õ¹Ē°¾°”£ŗ£Ė®ÖŠäåŌŖĖŲŅŌBr£ŠĪŹ½“ęŌŚ£¬¹¤ŅµÉĻÓĆæÕĘų“µ³ö·Ø“Óŗ£Ė®ÖŠĢįČ”äåµÄ¹¤ŅÕĮ÷³ĢČēĻĀĖłŹ¾£ŗ
(1)²½Öč¢Ł·“Ó¦µÄĄČ×Ó·½³ĢŹ½ĪŖ_______________________________”£
(2)²½Öč¢Ū·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________”£
(3)BrµÄŌ×ÓŠņŹżŹĒ________£¬ŌŚÖÜĘŚ±ķÖŠĪ»ÓŚµŚ________ÖÜĘŚ”¢________×唣
(4)²½Öč¢ŻÕōĮóµÄ¹ż³ĢÖŠ£¬ĪĀ¶ČÓ¦æŲÖĘŌŚ80”«90”ę”£ĪĀ¶Č¹żøß»ņ¹żµĶ¶¼²»ĄūÓŚÉś²ś£¬Ēė½āŹĶŌŅņ£ŗ________________”£
(5)ĪŖŹ²Ć“²»Ö±½ÓÓĆ”°äåĖ®»ģŗĻĪļ¢ń”±¶ųŅŖÓĆ”°äåĖ®»ģŗĻĪļ¢ņ”±½ųŠŠÕōĮó£¬µĆ³öŅŗäå£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
æ×ČøŹÆµÄÖ÷ŅŖ³É·ÖĪŖCu2(OH)2CO3”£Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§Éč¼Ę“Óæ×ČøŹÆÖŠŅ±Į¶ĶµÄ·½°øČēĻĀ£ŗ
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌŚ·ŪĖéŗóµÄæ×ČøŹÆÖŠ¼ÓČėĻ”ĮņĖį£¬¹Ū²ģµ½µÄĻÖĻóŹĒ_______________”£
·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ___________________________________________”£
(2)²Ł×÷aÖŠÓƵ½µÄ²£Į§ŅĒĘ÷ŹĒ____________________________________”£
(3)AµÄ»ÆѧŹ½ĪŖ________£¬ŌŚŠü×ĒŅŗÖŠ¼ÓČėAµÄÄæµÄŹĒ______________”£
(4)²Ł×÷b°üĄØĻ“µÓŗĶµĶĪĀŗęøÉ£¬×÷ÓĆŹĒ__________________________”£
(5)ÓŠĶ¬Ń§ČĻĪŖ£¬ĄūÓĆĢśŠ¼ŗĶĻ”ĮņĖį£¬²»ĶعżÉĻŹöŹµŃé·½°ø£¬Ņ²ÄÜ“Óæ×ČøŹÆÖŠŅ±Į¶Ķ”£ĒėÄćÓĆ¼ņ½ąµÄĪÄ×ÖĖµĆ÷²»Ķ¬·½°øµÄŹµŃéŌĄķ__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĀČ”¢ä唢µā”¢ÄĘ”¢Ć¾”¢ĆĢ”¢ļ®”¢ÓĖµČŌŖĖŲŌŚŗ£ŃóÖŠ±»ø»¼Æ”£ŗ£ŃóæŖ·¢ĄūÓĆŗĶĪ¬ČØŹĒ¹ś¼ŅÕ½ĀŌ”£
£Ø1£©ŅŌÉĻŌŖĖŲŌŚŗ£Ė®ÖŠµÄ“ęŌŚŠĪĢ¬ŹĒ___________£ØŃ”Ģī¢ŁÓĪĄėĢ¬¢Ś»ÆŗĻĢ¬¢Ū²»Č·¶Ø£©
£Ø2£©ŅŌĻĀ±ä»ÆŹōÓŚ»Æѧ±ä»ÆµÄŹĒ__________________________£ŗ
¢Ł ·¢ÉśŗĖĮŃ±ä£¬¢ŚÖŲĒā£Ø2H£©·¢ÉśŗĖ¾Ū±ä£¬¢ŪLiH×öŅ°ĶāÉśĒā¼Į£¬¢Üŗ£Ė®É¹ŃĪ
·¢ÉśŗĖĮŃ±ä£¬¢ŚÖŲĒā£Ø2H£©·¢ÉśŗĖ¾Ū±ä£¬¢ŪLiH×öŅ°ĶāÉśĒā¼Į£¬¢Üŗ£Ė®É¹ŃĪ
£Ø3£©ÓÉĀČĘųŗĶɹŃĪŗóµÄĀ±Ė®ÖĘČ”ŅŗäåµÄĄė×Ó·½³ĢŹ½______________________________£»ÓÉŹÆ»ŅČéŗĶĀ±Ė®³Įµķ³öĆ¾ŌŖĖŲµÄĄė×Ó·½³ĢŹ½_____________________________________£»ÓÉŗ£“ų»Ņæɽž³öKI£¬ÓÉijÖÖæóŃĪæɽž³öKIO3£¬¶žÕßŌŚŃĪĖįÖŠ·“Ó¦æÉÉś³Éµāµ„ÖŹ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½__________________________________”£
£Ø4£©¹¤ŅµÓÉĀČ»ÆÄĘÖĘČ”½šŹōÄʵĻÆѧ·½³ĢŹ½ĪŖ____________________________£»¹¤ŅµÖĘĆ¾²ÉÓƵē½āČŪČŚĀČ»ÆĆ¾£¬²»²ÉÓƵē½āČŪČŚŃõ»ÆĆ¾µÄŌŅņŹĒ_______________________
£Ø5£©ŗ£µ×ø»¼Æ¶ąÖÖæóĪļ½įŗĖ£¬ĆĢ½įŗĖŹĒĘäÖŠµÄŅ»ÖÖ”£ĆĢ½įŗĖÖŠÖ÷ŅŖŗ¬ÓŠMnO2ŗĶFe2O3”£Ņ»ÖÖÖŹĮæ±ČŌ¼ĪŖm(Mn):m(Fe)=55:448µÄŗĻ½šøÖ£ØĘäĖüŌŖĖŲĀŌ£©£¬¾ßÓŠæ¹Ół¼¤ĮŅ³å»÷ŗĶÄ„ĖšµÄÄÜĮ¦£¬æÉ×öŗ½Äø¼×°åµČ”£ÓūĶعżĀĮČČ·“Ó¦Į¶µĆÕāŃłµÄŗĻ½š£¬MnO2”¢Fe2O3”¢AlµÄĶ¶ĮĻ±Č£Ø°“ĪļÖŹµÄĮæÖ®±Č£©Ō¼ĪŖ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½¹ŃĒĮņĖįÄĘ£ØNa2S2O5£©³£ÓĆ×÷Ź³Ę·ĘÆ°×¼Į”£ĘäÖʱø¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ£ŗ·“Ó¦¢ņ°üŗ¬2NaHSO3 Na2S2O5£«H2OµČ¶ą²½·“Ó¦”£
Na2S2O5£«H2OµČ¶ą²½·“Ó¦”£
£Ø1£©ŹµŃéŹŅÖĘČ”°±ĘųµÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø2£©”°×ĘÉÕ”±Ź±·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©ŅŃÖŖNa2S2O5ÓėĻ”ĮņĖį·“Ó¦·Å³öSO2£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ£ŗ ”£
£Ø4£©ø±²śĘ·XµÄ»ÆѧŹ½ŹĒ£ŗ £»æÉŃ»·ĄūÓƵÄĪļÖŹŹĒ£ŗ_____________”£
£Ø5£©ĪŖĮĖ¼õÉŁ²śĘ·Na2S2O5ÖŠŌÓÖŹŗ¬Į棬ŠčæŲÖĘ·“Ó¦¢ņÖŠĘųĢåÓė¹ĢĢåµÄĪļÖŹµÄĮæÖ®±ČŌ¼
ĪŖ ”£
£Ø6£©¼ģŃé²śĘ·ÖŠŗ¬ÓŠĢ¼ĖįÄĘŌÓÖŹĖłŠčŹŌ¼ĮŹĒ £ØĢī±ąŗÅ£©
¢ŁĖįŠŌøßĆĢĖį¼Ų ¢ŚĘ·ŗģČÜŅŗ ¢Ū³ĪĒåŹÆ»ŅĖ® ¢Ü±„ŗĶĢ¼ĖįĒāÄĘČÜŅŗ ¢ŻNaOH ¢ŽĻ”ĮņĖį
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ½šŹōµÄ¹¤ŅµÖĘ·ØÕżČ·µÄŹĒ(””””)
| A£®ÖĘīŃ£ŗÓĆ½šŹōÄĘÖĆ»»³öĀČ»ÆīŃ(TiCl4)ČÜŅŗÖŠµÄīŃ |
| B£®Į¶Ģś£ŗÓĆ½¹ĢæŗĶæÕĘų·“Ó¦²śÉśµÄŅ»Ńõ»ÆĢ¼ŌŚøßĪĀĻĀ»¹ŌĢśæóŹÆÖŠµÄĢś |
| C£®ÖĘÄĘ£ŗÓĆŗ£Ė®×÷ŌĮĻÖĘµĆ¾«ŃĪ£¬ŌŁµē½ā“æ¾»ĀČ»ÆÄĘČÜŅŗµĆµ½½šŹōÄĘ |
| D£®Į¶Ķ£ŗÓĆ»ĘĶæó¾µē½ā¾«Į¶µĆµ½“æ¶ČĪŖ99.9%µÄĶ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com