
| A���춡��ת��Ϊ������Ĺ�����һ�����ȹ��� | B����������ȶ��Դ����춡�� | C����������Ӵ�������������춡����� | D���춡������е�̼�����������Ķ� |


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
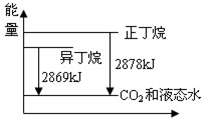 ��֪����ͬ������1mol�������1mol�춡����ȫȼ������CO2��Һ̬ˮ���ų���������ͼ��ʾ��������˵����ȷ���ǣ�������
��֪����ͬ������1mol�������1mol�춡����ȫȼ������CO2��Һ̬ˮ���ų���������ͼ��ʾ��������˵����ȷ���ǣ�������| A��1mol�������1mol�춡����ȫȼ�շų���������9 kJ | B����������Ӵ�������������춡�� | C���춡��ת��Ϊ������Ĺ�����һ�����ȹ��� | D���춡������е�̼������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ����һ�и߶���һ�ο��Ի�ѧ�Ծ����������� ���ͣ������
��6�֣�2008�걱�����˻����û��ȼ��Ϊ���飨C3H8����Ϥ����˻����û��ȼ��Ϊ65%���飨C4H10����35%���飨C3H8������֪������1mol����ȼ�շų�2220kJ������1mol������ȼ�շų�2878kJ������1mol�춡��ȼ�շų�2869.6kJ�������Իش��������⣺
��1����ʾ������ȼ�յ��Ȼ�ѧ��Ӧ����ʽ ��
��2�������й�˵����ȷ���� ��
| A�����˻��ȼ��ʱ������ת����Ҫ���ɻ�ѧ��ת��Ϊ���� |
| B����ͬ��������ͬ������������ͱ�����ȼ�գ��ų�������������ıȽ϶� |
| C����������춡�鲻�ȶ� |
| D���춡������е�̼�����������Ķ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com