
 X��Y��Z���ֳ���Ԫ�صĵ��ʣ��ס��������ֳ����Ļ�����������ͼת����ϵ���ش��������⣺
X��Y��Z���ֳ���Ԫ�صĵ��ʣ��ס��������ֳ����Ļ�����������ͼת����ϵ���ش��������⣺ ��Y��ԭ������Ϊ6�����̬ԭ�ӵĹ����ʾʽΪ
��Y��ԭ������Ϊ6�����̬ԭ�ӵĹ����ʾʽΪ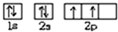 ��
�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ŵ� |
| ��� |
| A���ŵ�ʱ��������Ӧʽ��Pb+SO42--2e-�TPbSO4 |
| B���ŵ�һ��ʱ���������Χ��Һ���������� |
| C�����ʱ��������Ӧʽ��PbSO4+2H2O-2e-�TPbO2+4H++SO42- |
| D�����ʱ��Ҫʹ2mol PbSO4��ΪPb��PbO2��Ҫͨ��4mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | C-H | C-F | H-F | F-F |
| ���� | 414 | 489 | 565 | 158 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ȫ�������ܵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ����ͼ��ʾ������H+�������Dz���������Ӧ����ͨ������Ĥ�����ƶ�ʹ�Ҳ���Һ���ֵ����ԣ������й�˵������ȷ���ǣ�������
ȫ�������ܵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ����ͼ��ʾ������H+�������Dz���������Ӧ����ͨ������Ĥ�����ƶ�ʹ�Ҳ���Һ���ֵ����ԣ������й�˵������ȷ���ǣ�������| A���ŵ�ʱ�������Һ���ɻƱ�������缫��ӦʽΪ��VO2++e-+2H+�TVO2++H2O |
| B�����ʱ��ת�Ƶĵ�����Ϊ3.01��1023���������Һ��n��H+���ı仯��Ϊ1.0mol |
| C�����ʱ��H+����۶����ƶ����Ҳ� |
| D���������У��Ҳ���Һ��ɫ������ɫ��Ϊ��ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com