
| |||||||||||||||||||||||
(1) |
CO2(1��),NH3(1��) |
(2) |
Na+��AlO2����CO32��(ÿ��1�֣���3��) |
(3) |
CO32+��2H+����CO2����H2O(2��) AlO2����4H+����Al3+��2H2O(2��) |
(4) |
Al3+��3HCO3������Al(OH)3����3CO2��(2��) |
(5) |
SO42��(1��)�� �ڳ������м���������ϡ���ᣬ����ɫ��������ȫ�ܽ⣬����ҺX������SO42�����ӣ���������ȫ�ܽ⣬����ڸ����ӣ�(3��) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
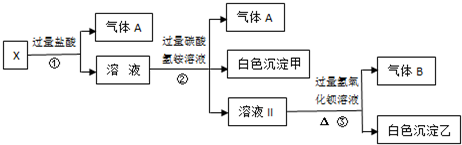
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��AlO![]() ��MnO
��MnO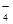 ��CO
��CO![]() ��SO
��SO![]() �е�������������ɣ�ȡ��Һ������������ʵ�飺
�е�������������ɣ�ȡ��Һ������������ʵ�飺
��1����ɫ�������� ��
��2��X��Һ��һ�����ڵ������� ��
��3����ɫ��������һ���У� �������� ֤�����Ƿ���ڵķ����� ��
��4����������������A������������Bͨ��ˮ�У�д����Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��AlO2-��MnO4-��CO32-��SO42-�е�������������ɣ�ȡ��Һ������������ʵ�飺
(1)��ɫ�������� _________��
(2)X��Һ��һ�����ڵ�������_____________��
(3)��ɫ��������һ���У�______��������_______ ֤�����Ƿ���ڵķ�����______________��
(4)��������������A������������Bͨ��ˮ�У�д����Ӧ�����ӷ���ʽ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ������ѧ�ڵ�һ���¿���ѧ�� ���ͣ������
ij��ɫ��ҺX����Na+ ��Ag+ ��Ba2+ ��Al3+ ��[Al(OH)4]-- �� MnO4����CO32-- ��SO42���е�������������ϣ�ȡ��Һ���������������飺���ѧ���
 1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________
1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________
2)X��Һ��һ�����ڵ������ǣ�____________________________
3)д������ٷ�����Ӧ���������ӷ�Ӧ����ʽ��L_________________________
4��д��������γɰ�ɫ���������ӷ���ʽ��______________________
5)д����ɫ�����ҵĿ�����ɣ�____________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com