
 �̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ�
�̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ�| t/min | 0 | 2 | 4 | 6 |
| V��O2��mL | 0 | 9.9 | 17.2 | 22.4 |
| ��Ӧ | ƽ�ⳣ��KP | |
| 773K | 873K | |
| ��CO2��g��+4H2��g��?CH4��g��+2H2��g�� | 19.4 | 0.803 |
| ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�� | 6.07��10-9 | 3.65��10-9 |
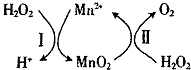
���� ��1��MnO��OH����OԪ�صĻ��ϼ�Ϊ-2�ۣ�HԪ�صĻ��ϼ�Ϊ+1�ۣ���ϻ��������������ϼ۵Ĵ�����Ϊ0���㣻
��2����ΪKsp��MnS��=1.4��10-15��Ksp��FeS��=6.0��10-18��Ksp��ZnS��=2.9��10 -25����ϵ�ػ�ԭ��ķ�Һ������Mn2+��Fe2+��Zn2+�ȣ�����εμ�Na2S��Һ�����������ܽ����С�����ʣ�
��3����ת����ϵ��֪����������������ӷ�Ӧ�õ��������̺������ӣ�
2H2O2��l���T2H2O��l��+O2��g����H1�٣�
MnO2��s��+H2O2��l��+2H+�TMn2+��aq��+O2��g��+2H2O��l����H2����
�ɸ�˹���ɢ�-�ڵõ�H2O2��l��+Mn2+��aq���TMnO2��s��+2H+��aq����H=��H1-��H2���Դ���д�Ȼ�ѧ����ʽ��
��0��2min���������������ʵ���n��O2��=$\frac{9.9��1{0}^{-3}L}{22.4L/mol}$=0.00044mol��n��H202��=2n��O2��=0.00088mol��v��H2O2��=$\frac{{\frac{0.00088mol}{0.01L}}}{2min}$��4.4��10-2mol/��L•min����4��6min���������������ʵ���n��O2��=$\frac{��22.4-17.2����1{0}^{-3}L}{22.4L/mol}$=2.3��10-4mol��n��H202��=2n��O2��=4.6��10-4mol��v��H202��=$\frac{{\frac{{4.6��{{10}^{-4}}mol}}{0.01L}}}{2min}$=2.3��10-2mol/L•min��0��2min H2O2ƽ����Ӧ���ʱ�4��6min�죬ԭ��Ϊ���ŷ�Ӧ�Ľ��У�H2O2Ũ�Ȳ��ϼ�С����Ӧ���ʲ��ϼ�����0��6min���������������ʵ���n��O2��=$\frac{{22.4��{{10}^{-3}}L}}{22.4L/mol}$=0.001mol��n��H202��=2n��O2��=0.002mol��v��H202��=$\frac{{\frac{0.002}{0.01}}}{6}$��3.3��10-2mol/��L•min����
��4�����¶���773K���ߵ�873K��ƽ�ⳣ����С�����������¶ȣ�ƽ�����淴Ӧ�����ƶ���
��ƽ�ⳣ��Ϊ������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ�
��� �⣺��1��MnO��OH����OԪ�صĻ��ϼ�Ϊ-2�ۣ�HԪ�صĻ��ϼ�Ϊ+1�ۣ������������ϼ۵Ĵ�����Ϊ0����Ԫ�صĻ��ϼ�Ϊ+3�ۣ��ʴ�Ϊ��+3��
��2����ΪKsp��MnS��=1.4��10-15��Ksp��FeS��=6.0��10-18��Ksp��ZnS��=2.9��10 -25����ϵ�ػ�ԭ��ķ�Һ������Mn2+��Fe2+��Zn2+�ȣ�����εμ�Na2S��Һ�����������ܽ����С�����ʣ��������ɵij���ΪZnS���ʴ�Ϊ��ZnS��
��3����ת����ϵ��֪����������������ӷ�Ӧ�õ��������̺������ӣ�
2H2O2��l���T2H2O��l��+O2��g����H1�٣�
MnO2��s��+H2O2��l��+2H+�TMn2+��aq��+O2��g��+2H2O��l����H2����
���ݸ�˹���ɢ�-�ڵõ�H2O2��l��+Mn2+��aq���TMnO2��s��+2H+��aq����H=��H1-��H2
�ʴ�Ϊ��H2O2��l��+Mn2+��aq���TMnO2��s��+2H+��aq����H=��H1-��H2
��0��2min���������������ʵ���n��O2��=$\frac{9.9��1{0}^{-3}L}{22.4L/mol}$=0.00044mol��n��H202��=2n��O2��=0.00088mol��v��H2O2��=$\frac{{\frac{0.00088mol}{0.01L}}}{2min}$��4.4��10-2mol/��L•min����4��6min���������������ʵ���n��O2��=$\frac{��22.4-17.2����1{0}^{-3}L}{22.4L/mol}$=2.3��10-4mol��n��H202��=2n��O2��=4.6��10-4mol��v��H202��=$\frac{{\frac{{4.6��{{10}^{-4}}mol}}{0.01L}}}{2min}$=2.3��10-2mol/L•min��0��2min H2O2ƽ����Ӧ���ʱ�4��6min�죬ԭ��Ϊ���ŷ�Ӧ�Ľ��У�H2O2Ũ�Ȳ��ϼ�С����Ӧ���ʲ��ϼ�����0��6min���������������ʵ���n��O2��=$\frac{{22.4��{{10}^{-3}}L}}{22.4L/mol}$=0.001mol��n��H202��=2n��O2��=0.002mol��v��H202��=$\frac{{\frac{0.002}{0.01}}}{6}$��3.3��10-2mol/��L•min����
�ʴ�Ϊ�������ŷ�Ӧ�Ľ��У�H2O2Ũ�Ȳ��ϼ�С����Ӧ���ʲ��ϼ�����3.3��10-2mol/��L•min����
��4�����¶���773K���ߵ�873K��ƽ�ⳣ����С�����������¶ȣ�ƽ�����淴Ӧ�����ƶ���˵������Ӧ�Ƿ��ȷ�Ӧ���ʴ�Ϊ�����ȣ�
�ڷ�Ӧ��ΪCO2��g��+3H2��g��?CH3OH��g��+H2O��g������ƽ�ⳣ������ʽΪK=$\frac{c��C{H}_{3}OH����c��{H}_{2}O��}{c��C{O}_{2}����{c}^{3}��{H}_{2}��}$���ʴ�Ϊ��K=$\frac{c��C{H}_{3}OH����c��{H}_{2}O��}{c��C{O}_{2}����{c}^{3}��{H}_{2}��}$��
���� ���⿼�黯ѧƽ����㡢���ܵ���ʼ�ƽ���ƶ��ȣ�Ϊ��Ƶ���㣬�����Ȼ�ѧ����ʽ����д����ѧƽ����ƶ�����ѧ��Ӧ���ʵļ�������֪ʶ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� |  | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
| �� | 800 | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 | 0.50 |
| �� | 800 | c1 | c2 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
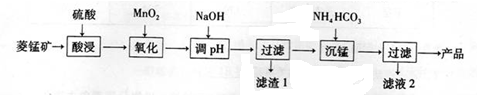
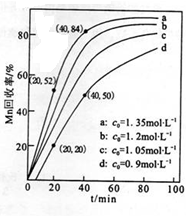
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ϡ���ᣬ��Һ��Ϊdz��ɫFe+4H++NO3 --=Fe3++NO��+2H2O | |
| B�� | ��K2Cr2O7��Һ�м�����Ũ���ᣬ��Һ��Ϊ��ɫCr2O7 2-����ɫ��+H2O?2CrO4 2-����ɫ��+2H+ | |
| C�� | ����۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ����4H++4I-+O2=2I2+2H2O | |
| D�� | ϡ�İ�ˮ��Һ�����������Ķ�����̼����NH3•H2O+CO2=NH4++HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��S2-�� CO32- | B�� | AlO21��SO42-����MnO4- | ||
| C�� | NO3-��Cl-��SO42- | D�� | MnO4-�� SO42-����NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����12.5% | B�� | ����12.5% | C�� | С��12.5% | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����-20���ɻ���ʹ�õ�̼��ά��һ�����͵��л��߷��Ӳ��� | |
| B�� | ȼ�ϵ����ȼ�϶��ڸ�������������Ӧ | |
| C�� | Fe3O4�׳����죬������ɫ�����Ϳ�� | |
| D�� | ��ҵ��ͨ���õ��Na��Mg��Al��Ӧ���Ȼ�����ȡ�����ֽ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
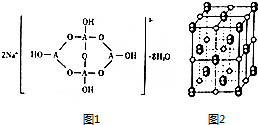 ��֪A��B��C��D��E����Ԫ�������ڱ���ǰ������Ԫ�أ���ԭ������������������A��B��CΪͬ���ڵķǽ���Ԫ�أ���B��Cԭ���о�������δ�ɶԵ��ӣ�D��EΪͬ����Ԫ���ҷֱ�λ��s����d��������Ԫ�����е�s�ܼ����Ӿ�Ϊȫ������E��d�ܼ�����������A��B��C����ܲ��p�ܼ�������֮�ͣ��ش��������⣺
��֪A��B��C��D��E����Ԫ�������ڱ���ǰ������Ԫ�أ���ԭ������������������A��B��CΪͬ���ڵķǽ���Ԫ�أ���B��Cԭ���о�������δ�ɶԵ��ӣ�D��EΪͬ����Ԫ���ҷֱ�λ��s����d��������Ԫ�����е�s�ܼ����Ӿ�Ϊȫ������E��d�ܼ�����������A��B��C����ܲ��p�ܼ�������֮�ͣ��ش��������⣺ ��
��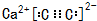 ���������ij����߷ֱ�Ϊ520pm��520pm��690pm���þ����ܶ�Ϊ2.28g/cm3��������С�������λ����
���������ij����߷ֱ�Ϊ520pm��520pm��690pm���þ����ܶ�Ϊ2.28g/cm3��������С�������λ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
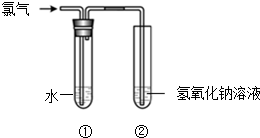 ijͬѧ����ͼ��ʾװ����ȡ��ˮ���������ʵ�飮
ijͬѧ����ͼ��ʾװ����ȡ��ˮ���������ʵ�飮�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com