
| t/min | 10 | 20 | 30 | 40 | 50 | 60 |
| n��CH3OH��/mol | 0.080 | 0.120 | 0.150 | 0.168 | 0.180 | 0.180 |
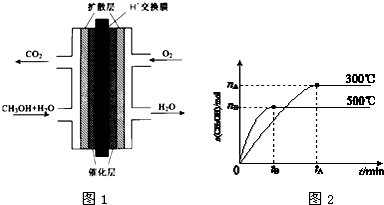
���� ��1���ɱ������ݿ�֪��0��20min��CH3OH�����ʵ����仯��Ϊ0.12mol������v=$\frac{\frac{��n}{V}}{��t}$����v��CH3OH��
��2���ɱ������ݿ�֪��50min����ƽ�⣬ƽ��ʱ�״������ʵ���Ϊ0.18mol���״�ƽ��Ũ��Ϊ$\frac{0.18mol}{2L}$=0.09mol/L����������ʽ����ƽ��ʱ����ֵ�Ũ�ȣ��ٸ���K=$\frac{c��C{H}_{3}OH��}{c��CO����{c}^{2}��{H}_{2}��}$����ƽ�ⳣ����
��3������ת����=$\frac{����Ũ�ȱ仯��}{������ʼŨ��}$��100%��
��4����ʵ����500���£�����ƽ��ʱn��CH3OH��=0.160mol��0.18mol��˵�������¶�ƽ�������ƶ���
��� �⣺��1���ɱ������ݿ�֪��300��ʱ��0��20min��CH3OH�����ʵ����仯��Ϊ0.12mol����v��CH3OH��=$\frac{\frac{0.12mol}{2L}}{20min}$=0.003mol/��L•min����
�ʴ�Ϊ��0.003��
��2����һ�ݻ�Ϊ2L�ĺ����ܱ������м���0.2mol CO��0.4mol H2��50min����ƽ�⣬ƽ��ʱ�״������ʵ���Ϊ0.18mol���״�ƽ��Ũ��Ϊ$\frac{0.18mol}{2L}$=0.09mol/L����
CO��g��+2H2��g��?CH3OH��g��
��ʼŨ�ȣ�mol/L����0.1 0.2 0
�仯Ũ�ȣ�mol/L����0.09 0.18 0.09
ƽ��Ũ�ȣ�mol/L����0.01 0.02 0.09
��ƽ�ⳣ��K=$\frac{c��C{H}_{3}OH��}{c��CO����{c}^{2}��{H}_{2}��}$=$\frac{0.09}{0.01��0.0{2}^{2}}$=2.25��104 ��
�ʴ�Ϊ��2.25��104 ��
��3������ת����=$\frac{0.18mol/L}{0.2mol}$��100%=90%��
�ʴ�Ϊ��90%��
��4����ʵ����500���£�����ƽ��ʱn��CH3OH��=0.160mol��0.18mol��˵�������¶�ƽ�������ƶ����������¶�ƽ�������ȷ�Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ�����ȣ�
���� ���⿼�黯ѧƽ�������Ӱ�����ء���Ӧ���ʼ��㡢ƽ�ⳣ���ȣ��ѶȲ���ע��Ի���֪ʶ���������գ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2Cl | B�� | CO2 | C�� | Cl2 | D�� | NaNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��Ӧʱ��/min | n��CO��/mol | n��H2O��/mol |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
| A�� | ��Ӧ��t1min�ڵ�ƽ������Ϊv��H2��=0.40/t1mol•L-1•min-1 | |
| B�� | ���������������䣬��ƽ����ϵ����ͨ��0.20molH2O����ԭƽ����ȣ��ﵽ��ƽ��ʱCOת��������H2O�����������С | |
| C�� | ���������������䣬��ʼʱ�������г���0.60molCO��1.20 molH2O������ƽ��ʱ��n��CO2��=0.40 mol | |
| D�� | �¶�����800�棬������Ӧƽ�ⳣ��Ϊ0.64��������ӦΪ���ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 |
| $\frac{P}{{P}_{0}}$ | 1.00 | 1.50 | 1.80 | 2.20 | 2.30 | 2.38 | 2.40 | 2.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������� | B�� | ���� | C�� | ����� | D�� | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com