
H2O2+2Fe2++2H+====2Fe3++2H2O
H2O2+2Fe3+====2Fe2++O2��+2H+
��1�������Ϸ�Ӧ��Fe2+ʵ��������_________���ã��ܷ�ӦʽΪ____________________��
��2��I2Ҳ��Fe2+һ�������������Ʒ�Ӧ����ȣ�1��������������ƽ�ĺ��ʵĻ�ѧ��Ӧ����ʽ��H2O2+I2====2HIO
__________________________________��
�ܷ�ӦʽΪ__________________________________��
��3����H2SO4��KI�Ļ����Һ�м���������H2O2���ų���������ɫ���壬��Һ����ɫ��������ʹ���۱�������ѧ����Ϊ�÷�Ӧ�����ӷ���ʽΪ��H2O2+2I-====I2+O2��+2H+���������ʽ��ȷ��__________________________________��
����ȷ�������ǣ�����Ϊ����ȷ���ò���������__________________��
������ȷ��ԭ���ǣ�����Ϊ��ȷ���ò���������__________________����д����ȷ�Ļ�ѧ��Ӧ����ʽΪ���������ӷ�Ӧ��д�����ӷ�Ӧʽ��û�����ӷ�Ӧ�ģ�д����ѧ��Ӧ����ʽ����_________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
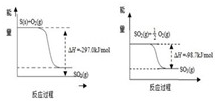
| 3 |
| 2 |
| 3 |
| 2 |
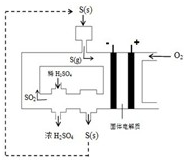
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
H2O2+2Fe2++2H+![]() 2Fe3++2H2O
2Fe3++2H2O
H2O2+2Fe3+![]() 2Fe2++O2��+2H+
2Fe2++O2��+2H+
H2O2�ĵ���ʽΪ______�������Ϸ�Ӧ��Fe2+ʵ��������______���ã��ܷ�Ӧ����ʽΪ____________��
��2��I2Ҳ��Fe2+һ�������������Ʒ�Ӧ����ȣ�1��������������ʵĻ�ѧ��Ӧ����ʽ��
H2O2+I2![]() 2HIO��______���ܷ�ӦʽΪ______��
2HIO��______���ܷ�ӦʽΪ______��
��3����H2SO4��KI�Ļ����Һ�м���������H2O2���ų���������ɫ���壬��Һ���ػ�ɫ��������ʹ���۱�������ѧ����Ϊ�÷�Ӧ�����ӷ���ʽΪH2O2+2I-![]() I2+O2��+2H+���������ʽ��ȷ�𣿣�������ȷ��ԭ���ǣ�����Ϊ��ȷ�����±�����______����д����ȷ�Ļ�ѧ��Ӧ����ʽ���������ӷ�Ӧ��д�����ӷ�Ӧʽ����______��
I2+O2��+2H+���������ʽ��ȷ�𣿣�������ȷ��ԭ���ǣ�����Ϊ��ȷ�����±�����______����д����ȷ�Ļ�ѧ��Ӧ����ʽ���������ӷ�Ӧ��д�����ӷ�Ӧʽ����______��
��4������H2O2��������KMnO4��Һ�У�������ɫ����ͬʱ��Һ����ɫ��dz���������ɣ�д����Ӧ�����ӷ���ʽ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����֪���з�Ӧ��һ�������¿��Է�����
H2O2+4Fe2++2H+ 2Fe3++2H2O H2O2+2Fe3+ 2Fe2++O2��+2H+
�����Ϸ�Ӧ��Fe2+ʵ�������� ���ã��ܷ�Ӧ
Ϊ ��
��2��I2��Fe2+һ��Ҳ�ܷ����������Ʒ�Ӧ����ȣ�1��д�����ʵĻ�ѧ��Ӧ����ʽ��
H2O2+I2 2HIO�� ���ܷ�ӦΪ ��
��3����H2SO4��KI�Ļ����Һ�м���������H2O2���ų���������ɫ���壬��Һ����ɫ��������ʹ������Һ��������ѧ����Ϊ�÷�Ӧ�����ӷ���ʽΪ��H2O2+2I�� I2+O2��+2H+���������ʽ��ȷ�� ������ȷ�������ǣ�����Ϊ����ȷ���ò��������� ��������ȷ��д����ȷ�Ļ�ѧ��Ӧ����ʽ���������ӷ�Ӧ��д�����ӷ�Ӧ����ʽ��û�����ӷ�Ӧ�ģ�д����ѧ��Ӧ����ʽ����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com