
(1)�ٴ�����Na2CO3 a g����Na2CO3��NaHCO3�Ļ����a g���۴�����NaHCO3 a g����Ҫ��ش��������⣺
A���ֱ���������ȫ��Ӧʱ���������Ӵ�С��˳��Ϊ__________��
B���ֱ��������ȫ��Ӧʱ���ų�CO2��������Ӵ�С��˳��Ϊ________________________________________________________________________��
C���ֱ�����ˮ���ټ��������ij���ʯ��ˮ�����ɳ����������Ӵ�С��˳��Ϊ________________________________________________________________________��
D���ֱ���ɵ��������Һ��c(Na��)��Ũ�ȴӴ�С��˳��Ϊ________________________________________________________________________��
(2)��a g Na2CO3��NaHCO3�Ļ�����ּ��ȣ���������Ϊb g����Na2CO3����������Ϊ__________��
(3)��������(1)�еĵ�������Ϊ�����ʵ������ش�����A��B��C��D���ʡ�
 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤������������������ȷ���� �� ��
A.28gC2H4�������õ��Ӷ���ĿΪ4NA B. 1L0.1mol��L-1������Һ��H+��Ϊ0.1NA
C. 1mol������������� ����Ϊ10NA D. ��״���£�22.4L�Ҵ��ķ�����ΪNA
����Ϊ10NA D. ��״���£�22.4L�Ҵ��ķ�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ͬ���������͵����������������Ĺ�ϵ�����ǣ�(1)ͬһԪ�صIJ�ͬԭ�ӣ�(2)��ͬԪ�ص�ԭ�ӣ�(3)���ֲ�ͬ�ķ��ӣ�(4)һ��ԭ�Ӻ�һ�ַ��ӣ�(5)һ��ԭ�Ӻ�һ�����ӣ�(6)һ�ַ��Ӻ�һ�����ӣ�(7)���ֲ�ͬ�������ӣ�(8)���ֲ�ͬ�������ӣ�(9)һ�������Ӻ�һ�������ӣ�������ȷ����
A��(1)(3)(4)(7)(8) B��(1)(2)(5)(7)(9) C��(1)(2)(5)(7)(9) D��(1)(2)(3)(4)(5)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һС������Ʒֱ�Ͷ��ʢaˮ��b�Ҵ���cϡH2SO4������С�ձ��У���Ӧ�����ɿ쵽����˳��Ϊ______________�����ͷ�Ӧ���ʲ�ͬ��ԭ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��Na2O2��CO2���ԡ�����ʵ���װ��ʾ��ͼ��
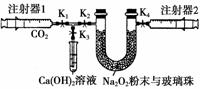
ע����1�г�ȡ��100 mL CO2��U�ι���װ������С�������Լ1.5 g Na2O2��ĩ��ʵ�鿪ʼʱ����K1��K2��K4ֹˮ�У��ر�K3ֹˮ�У��ƶ�ע����1������������CO2ѹ��U�ι��У�ע����2�������������ƶ����ش��������⣺(���ⲻ����ע�����ڱڵ�Ħ������)
(1)U�ι��ڵIJ����鲢�����뷴Ӧ������������ó���ֹ�������Ʒ�ĩ�������⣬��һ����Ҫ������________________________________________��
(2)U�ι��пɹ۲쵽�ķ�Ӧ������___________________________________________
________________________________________________________________________��
(3)ע����1�����Ƶ���ע����2���ռ������������50 mL����ԭ��������Ի���CO2�⣬����һ����Ҫԭ����
________________________________________________________________________
(4)Ϊ��ʹʣ�������ж�����̼�ܷ�Ӧ�꣬�������IJ�����
________________________________________________________________________��
(5)Ҫ֤����Ӧ����֮һΪ��������Ϊ�����ʵ�鷽����_______ _________________________________________________________________
_________________________________________________________________
________________________________________________________________________��
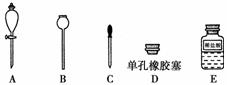
(6)��ʵ��������������ᣬ���Դ�U�ι���ȡ����Ϊ��Ҫ֤����������к���̼���Σ�ʵ��ʱ����Ҫ������ͼ��ʾ�������Լ���ѡ��________(����ĸ)����װ�ú�ҩƷ����װ��Ϻ������IJ���������____________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж���ɫ��Ӧʵ�����ע�������˵������ȷ����  (����)
(����)
�ټصĻ�����ɫҪ����ɫ�ܲ����۲졡���Ƚ���˿���յ���ԭ���������ɫ��ͬ����պȡ����������ʡ���ÿ��ʵ���Ҫ����˿������ϴ������ʵ��ʱ���ѡ������ɫ��dz�Ļ��桡��û�в�˿ʱ��Ҳ�����ù���������˿����
A�����Т۲���ȷ B�����Тܲ���ȷ
C�����Тݲ���ȷ D��ȫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���Ӧ�У������ﲻ�淴Ӧ������Ӧ��������仯���仯���� (����)
A��Na��O2 B��NaOH��CO2
C��NaHCO3��NaOH D��Na2CO3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ԫ��A��B��C��D��ԭ��������������A��C��ԭ�������IJ�Ϊ8��A��B��C����Ԫ��ԭ�ӵ�����������֮��Ϊ15��Bԭ����������������Aԭ��������������һ�롣����������ȷ���ǣ� ��
A��ԭ�Ӱ뾶��A��B��C��D B���ǽ����ԣ�A��C
C������������Ӧˮ��������ԣ�D��C D������B�ڳ�����������Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ����ѡ���װ�û�����(�г�װ������ȥ)��ȷ����
| A | B | C | D | |
| ʵ�� | ��CCl4��ȡ ��ˮ�е�Br2 | ��ȥ�Ҵ��еı��� | ��KI��I2�Ĺ��� ������л���I2 | ����100 mL 0.1000 mol��L��1 K2Cr2O7��Һ |
| װ�û����� |
|
|
|
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com