
���� ��1�����۲���ͨ����Ĥ���������ͨ����Ĥ��
��2��������������Ũ����ȣ�����NaCl������֪������Һ����������жϸ�Ĥ�ƶ�λ�ã���Ҫ������3����������Ũ��Ӧ��ȣ������ຬ��NaCl������ȣ�
��3�����ұ�Fe3+����Ĥ���������Һ���������·�Ӧ��2Fe3++SO2+2H2O=2Fe2++4H++SO42-��SO2�����ģ�Ʒ���ֱ��ͷų������ظ��������̣�
��4�����ɷ���ʽ��֪�������л�����������ʵ������䣻
�������������¶ȡ������ѹǿ��ͬ��ƽ��ʱ�����л��������ܵ����ʵ�����ͬ����ƽ��ʱ����������ʵ���Ϊ2.5mol��
����ʹ�Ҳ෴Ӧ��ʼʱV����V��������ʼʱ������������ʵ���Ӧ����2.5mol����B��ȫת��ʱB����ʼ���ʵ����K��ֵ��
��� �⣺��1����Ĥ����ˮ���ӡ�����������������������ͨ���İ�Ĥ����һ��ʱ����ұ�I2����ͨ����Ĥ������ߵ�����Һ������ߵ�����Һ��I2�������ұ���Һ��ɫ��dz��
�ʴ�Ϊ��C��B��
��2���ø�Ĥֻ����ˮ��������ͨ�����������һ�����������������Ũ����ȣ�����NaCl������֪������Һ���֮��Ϊa g��0.2a g=5��1����һ��ʱ����Ĥ���ջ��ƶ���5������Ҫ������3����������Ũ��Ӧ��ȣ������ຬ��NaCl������ȣ��������Ҳ���Һ�м���NaCl������NaCl������Ϊag-0.2ag=0.8a g��
�ʴ�Ϊ��5�����ұ���0.8agNaCl���壻
��3����ߵ�������Ʒ����Һ��SO2��Һ����Һ����ɫ���ұ�Fe3+����Ĥ���������Һ���������·�Ӧ��2Fe3++SO2+2H2O=2Fe2++4H++SO42-��SO2�����ģ�Ʒ���ֱ��ͷų������������Һ����ɫ���ɫ���ұ���Һ��ɫ��dz��
�ʴ�Ϊ��B��C��2Fe3++SO2+2H2O=2Fe2++4H++SO42-��
��4�����ɷ���ʽ��֪�������л�����������ʵ������䣬�ʴﵽƽ���Ӧ��������������ʵ���Ϊ2.5mol��
�ʴ�Ϊ��2.5��
�������������¶ȡ������ѹǿ��ͬ��ƽ��ʱ�����л��������ܵ����ʵ�����ͬ����ƽ��ʱ����������ʵ���Ϊ2.5mol��
����ʹ�Ҳ෴Ӧ��ʼʱV����V��������ʼʱ������������ʵ���Ӧ����2.5mol����0.5��3+x��2.5����x��1����B��ȫת��ʱB����ʼ���ʵ����K��ֵ��
��0.5-0.25x+0.5+0.5x+0.5+0.25x=2.5��
���x=2��
��x��ȡֵ��ΧΪ��1��x��2��
�ʴ�Ϊ��1��x��2��
���� ���⿼�黯ѧƽ����㡢��������ʡ���ҺŨ�ȵļ��㡢Ԫ�ػ��������ʵȣ����ؿ���ѧ������֪ʶ�������������������Ŀ�Ѷ��еȣ�


 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȳ���ʹ¯��˲��������ֲ��ܽ�ʡȼ�� | |
| B�� | �䲻��ʹ¯��˲������������Խ�ʡȼ�� | |
| C�� | ����ʹ¯��˲������ֿ��Խ�ʡȼ�� | |
| D�� | ���ܽ�ʡȼ�ϣ�����ʹ¯��˲����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
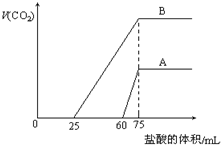 ȡ�����ݵ�Ũ�ȵ�NaOH��ҺA��B��ÿ��10mL���ֱ���A��B��ͨ�벻������CO2���ټ���������Һ����μ���0.2mol/L�����ᣬ��״���²�����CO2������������ӵ�������Һ���֮��Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
ȡ�����ݵ�Ũ�ȵ�NaOH��ҺA��B��ÿ��10mL���ֱ���A��B��ͨ�벻������CO2���ټ���������Һ����μ���0.2mol/L�����ᣬ��״���²�����CO2������������ӵ�������Һ���֮��Ĺ�ϵ��ͼ��ʾ���Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������м�������İ�ˮ��Al3++3OH-�TAl��OH��3�� | |
| B�� | �������������ᷴӦ��2Al��OH��3+6H+�T2Al3++6H20 | |
| C�� | ����ͭ������������Һ��Ӧ��Ba2++SO42-�TBaSO4�� | |
| D�� | ƫ��������Һ������ϡ���ᷴӦ��AlO2-+H++H20�TAl��OH��3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���з�Ӧ�ﵽƽ��ʱ��Q1=Q | |
| B�� | �ﵽƽ�����C������������Ҵ� | |
| C�� | �ﵽƽ����������м���0.25molA��0.75molB��1.5molC��ƽ��������C�ķ����ƶ� | |
| D�� | ���е��Ȼ�ѧ��Ӧ����ʽΪ2C��g��?A��g��+3B��g����H=+Q2kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | K+��Al3+��Ba2+��NH4+ |
| ������ | Cl-��OH-��SO42-��CO32- |
 ����Al3+��K+��SO42-�������ӿ����һ���Σ�д�����ε�һ����;��ˮ����
����Al3+��K+��SO42-�������ӿ����һ���Σ�д�����ε�һ����;��ˮ���� NH3•H2O+H+��
NH3•H2O+H+���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����Ȼ��Ϊԭ�Ϻϳɼ״������ķ�����ˮú������Ŀǰ���ڿ�����ֱ����������
����Ȼ��Ϊԭ�Ϻϳɼ״������ķ�����ˮú������Ŀǰ���ڿ�����ֱ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com