
�����״�(C6H5)3C-OH��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壮ʵ���Һϳ������״���ʵ��װ����ͼ��ʾ��
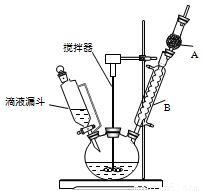
��֪���ٹ��������ɵ��м����ʸ����Լ�����ˮ�ⷴӦ��
�ڲ���������ʵķе����£�
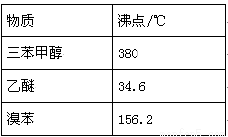
�������״�����Է�������Ϊ260��
��ش��������⣺
��1��װ���в�������B������Ϊ____________��װ����ˮCaCl2������A��������____________��
��2��װ���еμ�Һ��δ����ͨ��Һ©�����õ�Һ©����������____________��
��3���Ƶõ������״��ֲ�Ʒ�к������ѡ��屽���Ȼ�淋����ʣ�������������ᴿ������

���У������ٵ�������____________��ϴ��Һ���ѡ��____________(����ĸ���)��
a��ˮ b������ c���Ҵ� d����
�����Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊ____________��
��4�����Ȳⶨ����ȡ2.60g��Ʒ�����������Һ����������������(�������Ʋ���Ӧ)����ַ�Ӧ������ɵ������ڱ�״���µ����Ϊ100.80mL�����Ʒ�������״�����������Ϊ____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ����У��һ�µڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������̬��������干1L , �ڿ�������ȫȼ�յõ�1.5L CO2��2Lˮ����(�����������150�������²ⶨ), ���ڴ˻��������жϺ������ǣ� ��
A��һ�����м���
B��һ��������ϩ
C������������ͼ���Ļ������
D��һ��������ϩ����һ�����м���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016������ʡ������ѧ�ڵڰ˴��¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��PCl3��BCl3����������ԭ�ӵ�����㶼�ﵽ8�����ȶ��ṹ
B��Ϊ��ǿ��ˮ��Ư���ԣ����������м���̼���
C��NaH����ˮ��Ӧ�ķ���ʽ��NaH+D2O==NaOH+D2��
D��������NA�����ӵ�H2O��CH4������ͬ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��������������ѧԺ���и����¿�ǰ��ģ���ۻ�ѧ�Ծ��������棩 ���ͣ������
̼���仯����㷺��������Ȼ���У��ش��������⣺
��1������һ���ռ��˶�״̬�ĵ�����ԭ�Ӻ�����ֵĸ����ܶȷֲ�������������___________��
��2��̼���γɻ�����ʱ��������Թ��ۼ�Ϊ����ԭ����___________��
��3��C2H2 �����У����ۼ���������___________��C ԭ�ӵ��ӻ����������___________��д��������C2H2 ������ͬ�ռ乹�ͺ�̼������ӵķ���ʽ___________��
( 4 )CO �������Fe��Ni �ֱ��γ�Fe(CO)5��Ni(CO)4��Fe(CO)5 ��Fe Ԫ�ص�ԭ�Ӻ�������Ų�Ϊ______��Ni(CO)4 ����ɫҺ�壬�е�42.1�棬�۵�-19.3�棬������ˮ���������л��ܼ��Ʋ�Ni(CO)4 ��___________���塣
��5��̼�ж���ͬ�������壬����ʯīϩ����ʯ�ľ���ṹ��ͼ��ʾ��

����ʯīϩ�����У�ÿ��C ԭ������___________����Ԫ����ÿ����Ԫ��ռ��___________��C ԭ�ӣ�
���ڽ��ʯ�����У�C ԭ�������ӵ���С��ҲΪ��Ԫ����ÿ��C ԭ������___________����Ԫ������Ԫ���������___________��C ԭ����ͬһƽ�档
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��������������ѧԺ���и����¿�ǰ��ģ���ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�л���Ľṹ��ʽΪ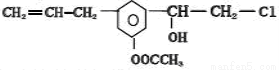 ����������˵����ȷ����( )
����������˵����ȷ����( )
A�����������ڷ�����
B��������������ˮ
C��1mol�������������2mol NaOH��Ӧ
D���������ܷ����ķ�Ӧ�����мӳɡ�ˮ�⡢��ȥ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ�����ڶ��ָ�ϰѵ������ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�����ʵ�����ƻ�����ܴﵽԤ��ʵ��Ŀ����( )
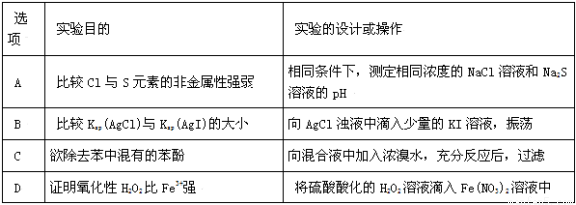
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ������ǰ����ģ�⻯ѧ�Ծ��������棩 ���ͣ������
�������γɶ��ֻ������NH3��N2H4��NH4NO3��NF3�ȡ�
��1����֪��N2(g)+2H2(g)�TN2H4(l)��H=+50.6kJ•mol-1
2H2(g)+O2(g)�T2H2O(l)��H=-571.6kJ•mol-1
��N2H4(l)+O2(g)�TN2(g)+2H2O ��H= _________kJ•mol-1��
��2����ˮ�е�NH4+����������þ�������������·�Ӧ��
MgO+H2O Mg(OH)2 Mg(OH)2+2NH4+
Mg(OH)2 Mg(OH)2+2NH4+ Mg2++2NH3•H2O
Mg2++2NH3•H2O
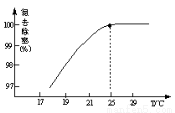
���¶ȶԵ������ʵ�Ӱ����ͼ��ʾ����25��ǰ�������¶ȵ�ȥ���������ԭ����________________��
��ʣ�������þ������Է�ˮ�γɶ�����Ⱦ��������________________��
��3����ˮ�еĺ��������ͨ����������Ĥ�ѵ����ս��д���������ϸ���������½�NH4+����ΪNO3-(2NH4++3O2�T2HNO2+2H2O+2H+��2HNO2+O2=2HNO3)��Ȼ�����״����״���NO3-��Ӧת��Ϊ���������壮
�����������У�14g�̬��Ԫ��ת��Ϊ��̬��Ԫ��ʱ����������Ϊ_______g��
��д������״���Ӧ�����ӷ���ʽ________________��
��4��ʹ��NaBH4Ϊ�յ�������ʹCo2+�����ڼ��������·�����Ӧ���Ƶøߴ��������ܣ��ù��̲������ж����塣
��д���÷�Ӧ�����ӷ���ʽ________________��
���������ܵĴ������£��¿ɷֽ������������壬����һ����ʹʪ��ĺ�ɫʯ����ֽ����������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ������������ͼ1��ʾ����N2H4�����ֽⷴӦ�Ļ�ѧ����ʽΪ________________��
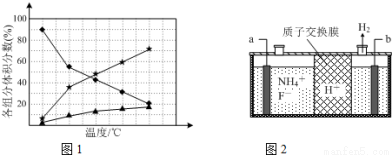
��5�������ӹ�ҵ��NF3�������������ʴ�̼�����ҵ��ͨ����⺬NH4F�ȵ���ˮ����������NF3������ԭ������ͼ2��ʾ��д��a�缫�ĵ缫��Ӧʽ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ������ǰ����ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ������������ɳ�����չ������ء�����˵������ȷ����
A������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ����������
B����ת��������β��Ϊ�����壬����������������ķ���
C���з���Ч�Ͷ���ũҩ�������߲˵�ũҩ������
D�����������ϵ�ص��ۺ����ü�������ֹ����е��ؽ�������Ⱦ������ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������������ѧ��У����һ��6�¿���ѧ���������棩 ���ͣ�ѡ����
NAΪ�����ӵ�������ֵ������˵����ȷ����( )
A����״���£�11.2L�����������ķ�����Ϊ0.5NA
B��0.5mol C3H8�����к�C��H���ۼ�2NA
C��11.2 L���ȼ�������������Ϊ0.5NA
D��������ϩ����ϩ����ϩ�Ļ�����干14 g����ԭ����Ϊ3NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com