
| | �ܽ��� | �е� |
| A | ����ˮ | 179.0�� |
| E | ������ˮ | 110.8�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
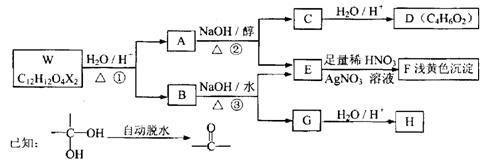
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
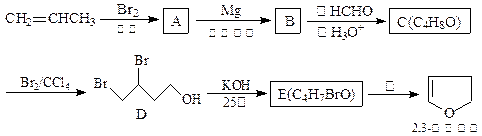
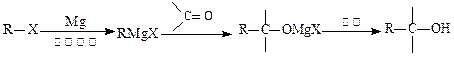
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��B�Ľṹ��ʽ ��
��B�Ľṹ��ʽ ��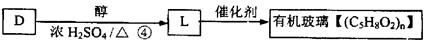
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
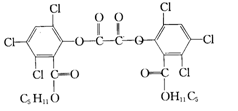
| A��CPPO���ڷ����廯���� |
| B��CPPO���ڸ߷��ӻ����� |
| C��1mol CPPO����������ϡ��Һ��Ӧ��������±�ز�ˮ�⣩���������4mol NaOH |
| D��1mol CPPO��������ȫ��Ӧ����Ҫ����10mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����ͼ��ʾ��ͼ��
����ͼ��ʾ��ͼ�� ������֮�����ߴ�����ѧ��������˫���ȡ���
������֮�����ߴ�����ѧ��������˫���ȡ���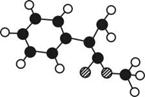
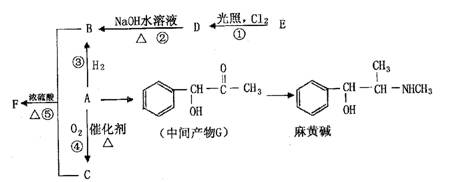
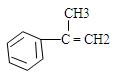 �������ϳ�A����ϳ�·�����£�
�������ϳ�A����ϳ�·�����£�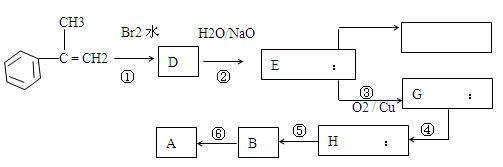
 Ӧ����ʽ��ע����Ҫ�ķ�Ӧ������
Ӧ����ʽ��ע����Ҫ�ķ�Ӧ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
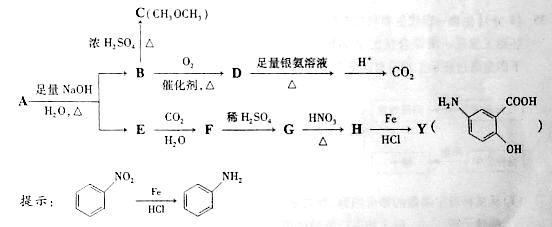
 C�ķ�Ӧ������____��
C�ķ�Ӧ������____��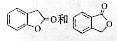 ������I��J���Լ�Ϊ ��
������I��J���Լ�Ϊ ��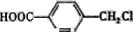 �Ƶã�д��K��Ũ�������������ɵľۺ���Ľṹ��ʽ_______________
�Ƶã�д��K��Ũ�������������ɵľۺ���Ľṹ��ʽ_______________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
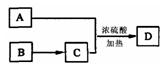
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
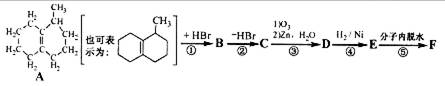
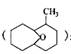 �⣬���õ�����ͬ���칹�壬
�⣬���õ�����ͬ���칹�壬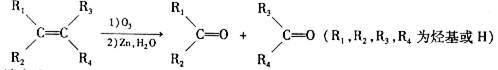
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com