

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��CO32-��OH
��CO32-��OH ��SO
��SO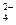 �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩.
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩. L-1 H2SO4 b��0.01mol
L-1 H2SO4 b��0.01mol L-1 KMnO4 c��1mol
L-1 KMnO4 c��1mol L-1 BaCl2
L-1 BaCl2| ʵ�鲽�� |  Ԥ������ͽ��� Ԥ������ͽ��� |
����1��ȡ��������Һ���Թ��У��μ�3 mol L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��С� L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��С� | |
| ����2����A�Թ��еμ�1��2�� ������ţ��� | ����Һ �������1������ ���������2��3������ |
| ����3����B�Թ��еμ�1��2�� ������ţ��� | ����Һ �������3������ ����ϲ���2�������2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
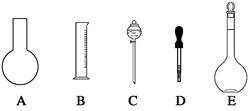
| A������һ�����ȷŨ�ȵı���Һ |
| B����ȡһ�������Һ�� |
| C����������ƿ������µ����������Һ�� |
| D��ȷϡ��ijһŨ�ȵ���Һ |
 ���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L��
���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
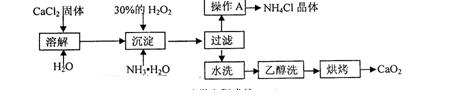
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
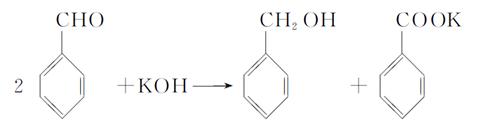
 ������ţ���
������ţ��� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
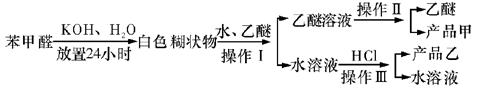
 ����ȡ������װ����ͼI�͢�
����ȡ������װ����ͼI�͢� ��
��| ������� | �ռ����� | ������� | ��֤���� | �ó����� |
| ��ˮ�к���������ʹʪ��ĺ�ɫ������ɫ�� | ��Cl2��ǿ������ ��Cl2����ˮ��Ӧ���������HClO ��HClO��ǿ������ | �� �� ������ʹ������ɫ�� �� �� ��H2Oʹ������ɫ | ��֤����٣��Ѻ�ɫ�ɲ��������Cl2�ļ���ƿ����������ɫ�� ��֤����ڣ� �� ��֤����ܣ��Ѻ�ɫ��������ˮ���������ɫ�� | ʹ��ɫ������ɫ�������� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com