
��10�֣�ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Ρ��ڷ�Ӧ�����Һ�У���μ���4mol��L��1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺

��1��DE�η�����Ӧ�����ӷ���ʽΪ��_____________________________________ ��
��2����д������һ��Al��Fe�뼫ϡHNO3��Ӧ�Ļ�ѧ����ʽ��_______________ ��
��3��B���Ӧ�ij��������ʵ���Ϊ_______mol��C���Ӧ������������Һ�����Ϊ______mL��
��4��ԭ������Һ�����ʵ���Ũ��Ϊ_______mol/L��
(10��,ÿ��2��)(1) NH4++OH��=NH3?H2O��
(2) 8Al+30HNO3=8Al(NO3)3+3NH4NO3+9H2O��8Fe+30HNO3=8Fe(NO3)3+3NH4NO3+9H2O
(3) 0.032�� 7��(4) 0.074��
��������
�����������1��Al��Fe����������ᷴӦ��������Ӧ�����Һ�м���NaOH��ʼ�����γɣ�˵�������������˽��������ᷴӦ����������������������OC�η�����Ӧ��H++ OH -= H2O����CD����Һ�е�Fe3+��Al3+����������Ӧ�γ�Fe(OH)3��Al(OH)3��������DE�Σ����������ʵ���û�б仯������Ϊ��DE��NaOH��Һ�����ᱻ��ԭΪNH4NO3,���߷������ֽⷴӦ�����ӷ���ʽ�ǣ�NH4++ OH-=NH3��H2O����2��8Al+30HNO3=8Al(NO3)3+3NH4NO3+9H2O��8Fe+30HNO3 =8Fe(NO3)3+3NH4NO3+ 9H2O����3����EF��NaOH�ܽ�Al(OH)3���������ӷ���ʽ�ǣ�Al(OH)3+ OH-= AlO2-+ 2H2O���ܽ�Al(OH)3���ĵ�NaOH�����ʵ�����n(NaOH)= 4mol/L�� 0.002L =0.008mol,���Ը��ݷ�Ӧ����ʽ�ж��ߵ����ʵ�����ϵ��֪��n(Al(OH)3)=0.008mol������Al(OH)3�������ĵ�NaOH��Һ�������6ml�����ݷ�Ӧ����ʽNH4++ OH-=NH3��H2O ��֪��n(NH4+)=4mol/L ��0.003L=0.012mol��n(e-)=0.012mol��8=0.096mol,Fe��Al����+3�۵Ľ����������ڷ�Ӧ�����е���ת����Ŀ��ȣ�����n(Fe)+n(Al)= 0.096mol��3=0.032mol,������B���Ӧ�ij��������ʵ�������������ʵ�����ȣ�n(����)= 0.032mol;ʹAl3+��Fe3+�γɳ������ĵ�NaOH�����ʵ�������ӵ����ʵ�����ȣ���0.096mol ����������NaOH��Һ�������V��NaOH��=0.096mol ��4mol/L=0.024L=24ml,���� C���Ӧ������������Һ�����Ϊ31ml-24ml=7ml����4�����ݷ���ʽH++ OH -= H2O��֪n(HNO3)=n(HNO3)(����)+ n(HNO3)(��Ӧ)= 4mol/L��0.007L+0.032mol��(30/8)=0.148mol.����ԭ�����Ũ����c(HNO3)= n(HNO3)��V=0.148mol��2.0L=0.074mol/L.
���㣺����Al��Fe�Ļ�ѧ���ʡ����ӷ���ʽ����д���غ㷨�ڻ�����и��ɷֵ�ȷ����Ӧ�õ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�����ʡ����10�µڶ��νο����Ծ��������棩 ���ͣ�ѡ����
NA��ʾ�����ӵ�����������˵����ȷ���ǣ� ��
A��16g CH4��18 g NH4+ �������������
B��1mol �������к���̼̼˫����Ϊ3NA
C������������ΪNA��NH3��HCl�Ļ���������ڱ�״���£������ΪԼ22. 4L
D�������ʵ����ļ�����CH3�����ǻ�����OH���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������Ƹ�ޭ���ص�һ����ѧ�߶������п��Ի�ѧ���������棩 ���ͣ�ѡ����
����˵������ȷ���ǣ� ��
A����ѿ�Ǽ���ˮ�������ܷ���������Ӧ
B������ˮ���ɼ��𱽷���Һ��2��4-�Ѷ�ϩ�ͼױ�
C�������������£�CH3CO18OC2H5��ˮ�������CH3CO18OH��C2H5OH
D���øʰ��ᣨH2NCH2COOH���ͱ����ᣨCH3CHNH2COOH�����������γ�4�ֶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���л�ѧ��Ӧ�����ӷ���ʽ��ȷ����
A��CuSO4��Һ��Ba(OH)2��Һ��ϣ�Ba2��+ SO2- 4= BaSO4��
B��̼������Һ�����ʯ��ˮ�ķ�Ӧ��CO2- 3��Ca2+ = CaCO3��
C���ô����ˮ����CaCO3��2H��=Ca2����H2O��CO2��
D������������Һ��ϡ���ᷴӦ��Ba2+ + SO2- 4 + H+ + OH- = BaSO4��+ H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ж��α���һ�����õ�ѧϰϰ�ߡ����տα�����������˵������ȷ����
A����������ʱ�����ܽ�����Լ�һ�μ���ȫ�������������
B������Դ�ḻ����ˮ��ȱ�Ĺ��ң������������ģ������ˮ
C�����շ�ɢ�ʻ��ɢ��״̬�IJ�ͬ�����ѷ�ɢϵ��Ϊ��Һ���������Һ����
D������ͬ���¶Ⱥ�ѹǿ�£��κ���������֮��ľ�����Կ�������ȵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ����ʮ�½��Կ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й�����Һ�ͽ������������ȷ����
A����Һ�ǵ����Եģ������Ǵ����
B��ͨ��ʱ����Һ�е��������ӷֱ��������ƶ��������еķ�ɢ��������ijһ���ƶ�
C����Һ���������ӵ��˶��й��ɣ������з�ɢ�����ӵ��˶����ɣ��������˶�
D��һ�����߷ֱ�ͨ����Һ�ͽ���ʱ������������ԵĹ����ǰ����û��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ����ʮ�½��Կ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ��,��Һ��ֻ����Na+��H+��Cl����OH������Һ������,��Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ
A��3��2��1 B��2��4��1 C��2��3��1 D��4��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ��У������ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ��ǣ� ��
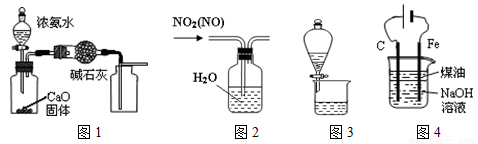
A����ͼ1װ����ȡ���ռ����﴿����NH3
B����ͼ2��ʾװ�ÿɳ�ȥNO2�е�NO
C����ͼ3��ʾװ�ÿɷ���CH3COOC2H5�ͱ���̼������Һ
D����ͼ4װ���Ʊ�Fe(OH)2���ܽϳ�ʱ��۲�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�캣��ʡ�������Ĵ��¿���ѧ�Ծ��������棩 ���ͣ������
����ͼ���ס��ҡ����ֱ��ʾ�ڲ�ͬ�����¿��淴Ӧ��A(g)+B(g)  xC(g)�������� C�ڷ�Ӧ������еİٷֺ�����C%���ͷ�Ӧʱ�䣨t���Ĺ�ϵ��
xC(g)�������� C�ڷ�Ӧ������еİٷֺ�����C%���ͷ�Ӧʱ�䣨t���Ĺ�ϵ��
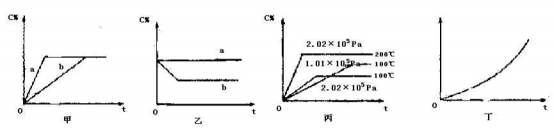
��1������ͼ���������߷ֱ�����д������������������ ���߱�ʾ����ʱ����������ʣ�a b������ڡ�С�ڻ���ڣ�
��2������ͼ��ʾ��Ӧ�ﵽƽ��ֱ��ں��º�ѹ�����ºͺ��º�����������ƽ���������г���He������������ ���߱�ʾ���º��ݵ��������ʱ�ú��º�����C% ������С�䣩
��3�����ݱ�ͼ�����жϸÿ��淴Ӧ������Ӧ�� ��Ӧ������ȡ����ȣ���������x��ֵ�� ��
��4����ͼ��ʾ��ij�̶��������ܱ������У��������淴Ӧ�ﵽƽ���ij�����������¶ȣ�T���ı仯���������������⣬��ͼ������������� ��[���C% ��A��ת���� ��B��ת���� ��ѹǿ ��c��A�� ��c(B)]������ƽ���ƶ��ķ���Ϊ ��������ƻ����ƣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com