
���� ��1������������Һ��һ�㲽��ѡ����Ҫ��������
��2������C=$\frac{n}{V}$��֪������ʹnƫ�������VƫС�IJ�������ʹ��ҺŨ��ƫ�ߣ�
��� �⣺��1����Ũ��������һ�����ʵ���Ũ��ϡ����һ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ�����������Ͳ����ͷ�ιܡ����������ձ�������ƿ������500mL0.2mol/L��ϡ����Ӧѡ��500mL����ƿ�������ò�������������ƿ��������ƽ��ҩ�ף�����Ҫ��������500mL����ƿ��
�ʴ�Ϊ���ڢޢߣ�500mL����ƿ��
��2������C=$\frac{n}{V}$��֪������ʹnƫ�������VƫС�IJ�������ʹ��ҺŨ��ƫ�ߣ�������Һ��δ��ȴ���̶��ݣ�������Һ���ƫС������ʱ���ӿ̶��ߣ�������Һ���ƫС��
�ʴ�Ϊ����Һ��δ��ȴ���̶��ݣ�����ʱ���ӿ̶��ߣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע��������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$�����������ķ�������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
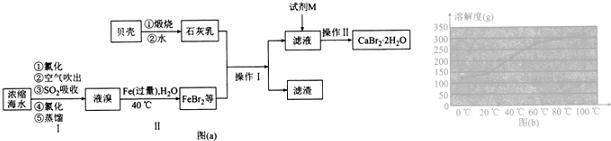
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
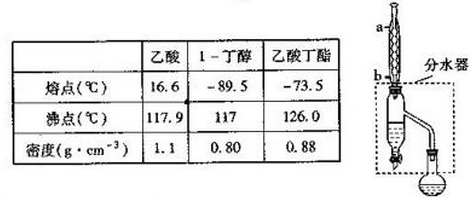

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 60% | B�� | 40% | C�� | 24% | D�� | 4% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
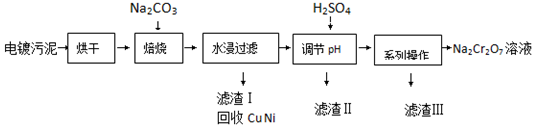
| 20�� | 60�� | 100�� | |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com