
���� ��1��A���Ȼ�����ǿ��ǿ���Σ�B�����������ᣬ��Һ�д��ڵ���ƽ�⣬C�����ᱵ��ǿ��ǿ���Σ�D����������ǿ��������ˮ��Һ��ˮ���Լ��ԣ�
������������ʵ�Ϊ B�����ᣬˮ��Һ�д��ڵ���ƽ�⣻
��D����������ǿ��������ˮ��Һ��ˮ���Լ��ԣ�
��2��ˮ�ĵ��������ȹ��̣�����ƽ��������У����������ӻ�����Ϊ10-14�����´ٽ�ˮ�ĵ��룬���ӻ���������
��� �⣺��1��������������ʵ���B�����ᣬˮ��Һ�д��ڵ���ƽ��CH3COOH?CH3COO-+H+��
�ʴ�Ϊ��B��CH3COOH?CH3COO-+H+��
�ڴ�������ǿ��������ˮ��Һ��ˮ���Լ��ԣ�ˮ�����ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-����Һ������Ũ�ȴ�СΪ��c��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ�����ԣ�CH3COO-+H2O?CH3COOH+OH-��c��Na+����c��CH3COO-����c��OH-����c��H+����
��2�����������ӻ�����Ϊ10-14�����´ٽ�ˮ�ĵ��룬���ӻ���������ij�¶�ˮ�����ӻ�KW=1��10-12��10-14��˵�����¶ȸ���25��C��
�ʴ�Ϊ������
���� ���⿼��������ˮ���ԭ���������������Һ������Ũ�ȴ�С�Ƚϣ�Ӱ��ˮ����ƽ��������жϵ�֪ʶ����Ŀ�ѶȲ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ��Ӧ�¶ȣ��棩 | �μӷ�Ӧ������ | ||||
| Na2S2O3 | H2SO4 | H2O�������mL�� | ||||
| �����mL�� | Ũ�ȣ�mol•L-1�� | �����mL�� | Ũ�ȣ�mol•L-1�� | |||
| A | 10 | 5.0 | 0.10 | 10.0 | 0.10 | 5.0 |
| B | 10 | 5.0 | 0.10 | 5.0 | 0.10 | 10.0 |
| C | 30 | 5.0 | 0.10 | 5.0 | 0.10 | 10.0 |
| D | 30 | 5.0 | 0.20 | 5.0 | 0.10 | 10.0 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a=b=100 | B�� | a=b=1000 | C�� | a��b | D�� | a��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
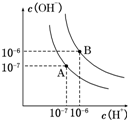 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ij���ܵ�ص�ԭ������ͼ��ʾ����Һ��c��H+��=2.0mol/L��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ۵ĵ缫��ӦΪV3++e-=V2+������������ȷ���ǣ�������
ij���ܵ�ص�ԭ������ͼ��ʾ����Һ��c��H+��=2.0mol/L��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ۵ĵ缫��ӦΪV3++e-=V2+������������ȷ���ǣ�������| A�� | �ŵ�ʱ�����·�ĵ�����a������b�� | |
| B�� | �ŵ�ʱ����Һ��H+����������Ҳ� | |
| C�� | ���ʱ��a���ķ�ӦʽΪVO2+-e-+H2O=VO2++2H+ | |
| D�� | �������Һ��ɫ����ɫ��Ϊ��ɫʱ�����������ת����ʽΪ��ѧ��ת��Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol�κ�����������Ϊ22.4 L | |
| B�� | 1 mol�κ������ڱ�״������ռ�������Ϊ22.4 L | |
| C�� | ��״���£�1 mol���Ȼ�̼��ռ�������22.4 L | |
| D�� | ��״���£�22.4 L���κ���������ʵ�������1 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | īˮ | B�� | Fe��OH��3���� | C�� | CuSO4��Һ | D�� | ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
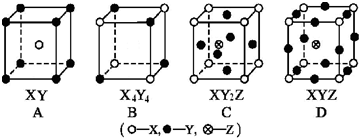
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com