
����˵���������
A�������£��������������Ļ����ҺpH=7����C(Na+)=c(CH3COO-)
B��ij�¶��£�pH=6��NaCl��Һ������Ũ�ȵĴ�С��ϵ��c(Na+)= c(Cl-)>c(H+)>c(OH-)
C����2a mol��L��1HCN��a mol��L��1 NaOH��Һ�������Ϻ�������Һ��c(Na��)>c(CN��)��������ҺpH>7
D����0.1 mol NaHCO3��0.2molNa2CO3�Ļ��Һ�У�c(Na��)��c(H��)��c(OH��)��c(HCO3��)��2c(CO32��)

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W������X��Z��W����Ԫ�ؿ����XH3��H2Z��HW���ۻ����Y����Ԫ�ؿ����Y2O�����ӻ�����Y2O2��
(1)д��Y2O2�ĵ���ʽ____________________________________________________��
���к��еĻ�ѧ����______________________________________________��
(2)�õ���ʽ��ʾZ��Y�γɻ�����Ĺ���______________________________________
______________________��
(3)XH3��H2Z��HW���ֻ��������һ�����������ֶ��ܷ�Ӧ����________(��д����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ˮ�ӵ�������KI��Һ�У��ټ���CCl4���ã�������������ȷ����(����)
A���õ����ȵ���ɫ��Һ
B����ɫˮ�����гȺ�ɫҺ��
C����ɫˮ��������ɫ��״Һ��
D����ɫˮ��������ɫ��״Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
H2��Cl2�ڵ�ȼ����������¶��ܷ�����Ӧ��
(1)H2��Cl2��ȼ��ʱ������Ϊ______________________________________��
�÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________
________________________________________________________________________��
�÷�ӦΪ________��Ӧ(����ȡ������ȡ�)��
(2)��֪�Ͽ�1 mol H2�еĻ�ѧ��������436 kJ���������Ͽ�1 mol Cl2�еĻ�ѧ��������243 kJ�����������γ�1 mol HCl�����еĻ�ѧ���ͷ�431 kJ������������1 mol H2��1 mol Cl2��Ӧ�������仯Ϊ________kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.10mol��L��1������ζ�0.10mol��L��1�İ�ˮ���ζ������в����ܳ��ֵĽ����
A��c(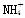 )>c(Cl��)��c(OH��)>c(H��) B��c(
)>c(Cl��)��c(OH��)>c(H��) B��c(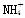 )��c(Cl��)��c(OH��)��c(H
)��c(Cl��)��c(OH��)��c(H
C��c(Cl��)>c(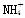 )��c(OH��)>c(H��) D��c(Cl��)>c(
)��c(OH��)>c(H��) D��c(Cl��)>c(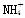 )��c(H��)>c(OH��)
)��c(H��)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I. ���ᣨH3PO4����ˮ��Һ�и��ִ�����ʽ���ʵ�����������pH�ı仯��������ͼ��
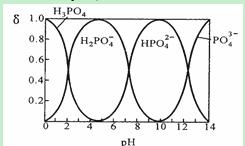
�ٴ�ͼ���ƶ�NaH2PO4��Һ�� ��(��ᡱ��������С�)����˵�� ��
����Na3PO4��Һ�У�c(Na+)/c(PO43��) 3���>����=����<�����������Һ�е��뼸��ŨKOH��Һ��c(Na+)/c(PO43-)��ֵ��С��ԭ���� ��
���ǻ���ʯ[Ca5(PO4)3OH]��һ����Ҫ�����������ϣ��йط�ӦΪ��
5Ca(OH)2��3H3PO4��Ca5(PO4)3OH��9H2O����ͼ�������ǻ���ʯʱ�õ���ʵ�����ߣ����������ij�ʼŨ��Ϊ0.70mmol/L��pH=10.0�����£���Ӧǰ10min������ij������� ��

II. ��ij�¶��£���1.0mol NH3����ˮ�����1L��Һ�������Һ��OH��Ũ�Ⱥ�ʱ���ͼ�����£�
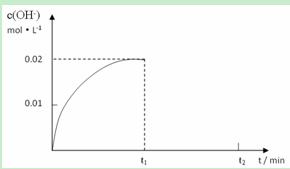
������¶�ʱ����ˮ�ĵ���ƽ�ⳣ��KΪ���٣�
����t1ʱ��ʱ�ټ���H2O���2L��Һ����t2ʱ��ʱ���´ﵽƽ�⣬��������ϵ�л���t1 ��t2ʱ����OH��Ũ����ʱ��仯�����ߡ�
�۽�a mol/L�������b mol/L��ˮ�������ϣ�������Һ�����ԣ����Ի��ǰ����Һ����ı仯�������Ϻ���Һ�У�c(NH3��H2O)�� �����ú���a��b��ʽ�ӱ�ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
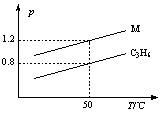
��ͼ���������߷ֱ��ʾ1 g C3H6��1 g M��������ͬ�����������ѹǿ���¶ȵĹ�ϵ���Ծ�ͼ���ж�M���������
A��SO2 B��CO C��C3H8 D��Cl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʣ����ռ���壻��ͭ˿��
��NH3����ϡ����ݶ�����̼���壻��ʳ��ˮ����̼���Ʒ�ĩ����ƾ����������Ȼ��ƣ���������������գ�
(1)����״̬�µ����ʿɵ������___________________________________��
(2)���ڵ���ʵ���____________________________________________��
(3)���ڷǵ���ʵ���_____________________________________��
(4)���ڵ���ʣ���������״̬�²��ܵ������____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com