
A.ΩœΕ®”–K+ΓΔ![]() ΓΔ
ΓΔ![]() B.ΩœΕ®”–Al3+ΓΔMg2+ΓΔ
B.ΩœΕ®”–Al3+ΓΔMg2+ΓΔ![]()
C.ΩœΕ®”–Al3+ΓΔMg2+ΓΔ![]() D.ΩœΕ®”–Al3+ΓΔMg2+ΓΔ
D.ΩœΕ®”–Al3+ΓΔMg2+ΓΔ![]() ΓΔCl-
ΓΔCl-
 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ


≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
Θ®8Ζ÷Θ©”–“ΜΈό…Ϊ»ή“ΚΘ§Τδ÷–Ω…ΡήΚ§”–Fe3+ΓΔAl3+ΓΔFe2+ΓΔMg2+ΓΔCu2+ΓΔNH4+ΓΔK+ΓΔCO32Θ≠ΓΔSO42Θ≠Β»άκΉ”ΒΡΦΗ÷÷Θ§ΈΣΖ÷ΈωΤδ≥…Ζ÷Θ§»Γ¥Υ»ή“ΚΖ÷±πΫχ––ΝΥΥΡΗω Β―ιΘ§Τδ≤ΌΉςΚΆ”–ΙΊœ÷œσ»γœ¬ΆΦΥυ ΨΘΚ
Θ®1Θ©‘Ύ‘≠»ή“Κ÷–“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ””– ΘΜ
Θ®2Θ©ΒΎΔέΗω Β―ι÷–Θ§»τ»Γ‘≠»ή“ΚΒΡΧεΜΐΈΣ100 mLΘ§ΒΈΦ”ΒΡNaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ0.5 molΓΛLΘ≠1 «“…ζ≥…ΑΉ…Ϊ≥ΝΒμΒΡΝΩ”κΦ”»κNaOHΒΡΝΩ”–”“ΆΦΥυ ΨΒΡœύΜΞΙΊœΒΘ§‘ρΗΟ»ή“Κ÷–ΥυΚ§―τάκΉ”ΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2010ΡξΚ”±± ΓΈΒœΊ“Μ÷–ΗΏ“Μœ¬―ßΤΎΤΎΡ©ΩΦ ‘Μ·―ß ‘Χβ Χβ–ΆΘΚΧνΩ’Χβ
Θ®8Ζ÷Θ©”–“ΜΈό…Ϊ»ή“ΚΘ§Τδ÷–Ω…ΡήΚ§”–Fe3+ΓΔAl3+ΓΔFe2+ΓΔMg2+ΓΔCu2+ΓΔNH4+ΓΔK+ΓΔCO32Θ≠ΓΔSO42Θ≠Β»άκΉ”ΒΡΦΗ÷÷Θ§ΈΣΖ÷ΈωΤδ≥…Ζ÷Θ§»Γ¥Υ»ή“ΚΖ÷±πΫχ––ΝΥΥΡΗω Β―ιΘ§Τδ≤ΌΉςΚΆ”–ΙΊœ÷œσ»γœ¬ΆΦΥυ ΨΘΚ






 Θ®1Θ©‘Ύ‘≠»ή“Κ÷–“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ””– ΘΜ
Θ®1Θ©‘Ύ‘≠»ή“Κ÷–“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ””– ΘΜ
Θ®2Θ©ΒΎΔέΗω Β―ι÷–Θ§»τ»Γ‘≠»ή“ΚΒΡΧεΜΐΈΣ100 mLΘ§ΒΈΦ”ΒΡNaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ0.5 molΓΛLΘ≠1«“…ζ≥…ΑΉ…Ϊ≥ΝΒμΒΡΝΩ”κΦ”»κNaOHΒΡΝΩ”–”“ΆΦΥυ ΨΒΡœύΜΞΙΊœΒΘ§‘ρΗΟ»ή“Κ÷–ΥυΚ§―τάκΉ”ΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2011-2012―ßΡξ…¬Ές ΓΈΦΡœ –ΗΏ»ΐΒΎΕΰ¥Έ÷ ΝΩΦλ≤βΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®10Ζ÷Θ©”–“ΜΆΗΟς»ή“ΚΘ§Ω…ΡήΚ§”– Β»άκΉ”÷–ΒΡ“Μ÷÷ΜρΦΗ÷÷ΓΘœ÷Φ”»κNa2O2ΖέΡ©÷Μ”–Έό…ΪΈόΈΕΒΡΤχΧεΖ≈≥ωΘ§≤ΔΆ§ ±Έω≥ωΑΉ…Ϊ≥ΝΒμΓΘ»γΦ”»κNa2O2ΒΡΝΩ”κ…ζ≥…ΑΉ…Ϊ≥ΝΒμΒΡΝΩ÷°ΦδΒΡΙΊœΒ”Οœ¬ΆΦά¥±μ ΨΓΘ
Β»άκΉ”÷–ΒΡ“Μ÷÷ΜρΦΗ÷÷ΓΘœ÷Φ”»κNa2O2ΖέΡ©÷Μ”–Έό…ΪΈόΈΕΒΡΤχΧεΖ≈≥ωΘ§≤ΔΆ§ ±Έω≥ωΑΉ…Ϊ≥ΝΒμΓΘ»γΦ”»κNa2O2ΒΡΝΩ”κ…ζ≥…ΑΉ…Ϊ≥ΝΒμΒΡΝΩ÷°ΦδΒΡΙΊœΒ”Οœ¬ΆΦά¥±μ ΨΓΘ
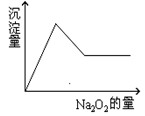
‘ΆΤΕœΘΚ‘≠»ή“Κ÷–“ΜΕ®Κ§”– ΘΜ“ΜΕ®ΟΜ”– Θ§Ω…ΡήΚ§ Θ§ΈΣΝΥΫχ“Μ≤Ϋ»ΖΕ®Ω…ΡήΚ§”–ΒΡάκΉ” Θ§”Π‘ωΦ”ΒΡ Β―ι≤ΌΉςΈΣ Θ§œ÷œσΈΣ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2010ΡξΚ”±± ΓΗΏ“Μœ¬―ßΤΎΤΎΡ©ΩΦ ‘Μ·―ß ‘Χβ Χβ–ΆΘΚΧνΩ’Χβ
Θ®8Ζ÷Θ©”–“ΜΈό…Ϊ»ή“ΚΘ§Τδ÷–Ω…ΡήΚ§”–Fe3+ΓΔAl3+ΓΔFe2+ΓΔMg2+ΓΔCu2+ΓΔNH4+ΓΔK+ΓΔCO32Θ≠ΓΔSO42Θ≠Β»άκΉ”ΒΡΦΗ÷÷Θ§ΈΣΖ÷ΈωΤδ≥…Ζ÷Θ§»Γ¥Υ»ή“ΚΖ÷±πΫχ––ΝΥΥΡΗω Β―ιΘ§Τδ≤ΌΉςΚΆ”–ΙΊœ÷œσ»γœ¬ΆΦΥυ ΨΘΚ


 Θ®1Θ©‘Ύ‘≠»ή“Κ÷–“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ””–
ΘΜ
Θ®1Θ©‘Ύ‘≠»ή“Κ÷–“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ””–
ΘΜ
Θ®2Θ©ΒΎΔέΗω Β―ι÷–Θ§»τ»Γ‘≠»ή“ΚΒΡΧεΜΐΈΣ100 mLΘ§ΒΈΦ”ΒΡNaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ0.5 molΓΛLΘ≠1 «“…ζ≥…ΑΉ…Ϊ≥ΝΒμΒΡΝΩ”κΦ”»κNaOHΒΡΝΩ”–”“ΆΦΥυ ΨΒΡœύΜΞΙΊœΒΘ§‘ρΗΟ»ή“Κ÷–ΥυΚ§―τάκΉ”ΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ ΓΘ

≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com