
ijһ��θҩ�е������Ϊ̼���,��������������������IJⶨ����:
��������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ
��ȡһ��(ҩƬ������ͬ)0.2 g�Ĵ�θҩƬ,ĥ������20.0 mL����ˮ
���Է�̪Ϊָʾ��,��0.1 mol��L-1��NaOH��Һ�ζ�,��ȥV mL��ζ��յ�
������25 mL 0.1 mol��L-1��HCl��Һ
(1)д��ʵ����̵IJ���(д���˳��)������������������������
(2)��ͼ��ʾ����������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ�϶�����Ҫ��������(�����)������,����������Һ����Ҫ�IJ���������������(����������)��
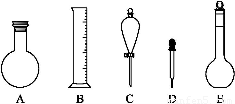
(3)����������ҺӦѡ�õ�����ƿ�����(����ĸ)������������
A.50 mL��50 mL
B.100 mL��100 mL
C.100 mL��150 mL
D.250 mL��250 mL
(4)д���йصĻ�ѧ��Ӧ����ʽ:����������������������������������
(5)ÿ��θҩ�к�̼��Ƶ�����Ϊ������ g��
 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��1 2.2��ȷʹ��ҩ����ϰ���������棩 ���ͣ�ʵ����
ijһ��θҩҩƬ�������Ϊ̼��ƣ���������������������IJⶨ���£�
��������0.1 mol/L��HCl��Һ��0.1 mol/L��NaOH��Һ��
��ÿ��ȡһ��(ҩƬ��������ͬ)0.2 g�Ĵ�θҩƬ��ĥ������20 mL����ˮ��
���Է�̪Ϊָʾ������0.1 mol/L��NaOH��Һ�ζ�������ȥVx mL�ﵽ�ζ��յ㣻
�ܼ���25 mL 0.1 mol/L��������Һ��
(1)д��ȫ��ʵ����̵IJ���˳��________(д���˳��)��
(2)��ͼ��ʾ����������0.1 mol/L��HCl��Һ��0.1 mol/L NaOH��Һ�϶�����Ҫ��������________(�����)������������Һ����Ҫ�õ��IJ���������________(����������)��
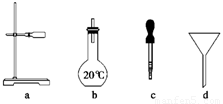
(3)����������ҺӦѡ�õ�����ƿ���ֱ�Ϊ________(����ĸ)��
A��50 mL,50 mL B��100 mL,100 mL
C��100 mL,150 mL D��250 mL,250 mL
(4)д����Ӧ�Ļ�ѧ����ʽ____________________________________________��
(5)θҩ�к�CaCO3������________g(д������ʽ����������˵��)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com