
��12�֣�A����;���Ľ�����B��C�����ֳ������嵥�ʣ�E��ҺΪ����ǿ�ᣬD��Һ�еμ�KSCN��Һ�Ժ�ɫ�������ת����ϵ��ͼ��ʾ��
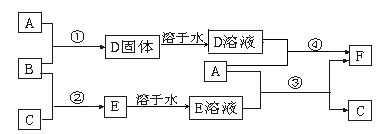
��ش�
��1��д��B���ʵĻ�ѧʽ�� ��F�����ƣ� ��
��2��д���ڢ۲���Ӧ�Ļ�ѧ����ʽΪ ��
��3���ڢܲ���Ӧ����Һ��ɫ�ı仯 ��
д���ڢܲ���Ӧ�����ӷ���ʽ ��
��4��д��SO2��B��Ӧ�����ӷ���ʽ ��
��5��F��Һ�е���NaOH��Һ���ܲ�����ʵ�������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��20111ѧ��ӱ�ʡ��ˮ��ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
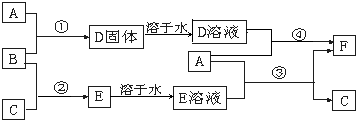
��12�֣�A����;���Ľ�����B��C�����ֳ������嵥�ʣ�E��ҺΪ����ǿ�ᣬD��Һ�еμ�KSCN��Һ�Ժ�ɫ�������ת����ϵ��ͼ��ʾ��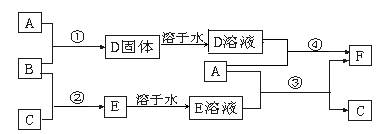
��ش�
��1��д��B���ʵĻ�ѧʽ�� ��F�����ƣ� ��
��2��д���ڢ۲���Ӧ�Ļ�ѧ����ʽΪ ��
��3���ڢܲ���Ӧ����Һ��ɫ�ı仯 ��
д���ڢܲ���Ӧ�����ӷ���ʽ ��
��4��д��SO2��B��Ӧ�����ӷ���ʽ ��
��5��F��Һ�е���NaOH��Һ���ܲ�����ʵ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012����Ϻ�����У��һ��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ������
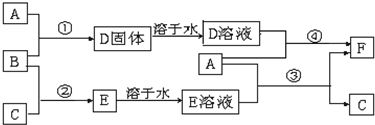
A����;���Ľ�����B��C�����ֳ������嵥�ʣ�E��ҺΪ����ǿ�ᣬD��Һ�еμ�KSCN��Һ�Ժ�ɫ�������ת����ϵ��ͼ��ʾ��

��ش�
��1��д��B���ʵĻ�ѧʽ�� ��F�����ƣ� ��
��2��д���ڢ۲���Ӧ�Ļ�ѧ����ʽΪ ��
��3���ڢܲ���Ӧ����Һ��ɫ�ı仯 ��
��4��F��Һ�е���NaOH��Һ���ܲ�����ʵ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com