
��֪A��B��C��DΪ��ѧ���������嵥�ʣ��ס��ҡ�������Ϊ�����Ļ������ˮ��Һ�ʼ��ԡ�����֮������ͼ��ʾ��ת����ϵ�����ֲ��P��Ӧ��������ȥ����

��ش�
 ��1���ҵ�����Ϊ ����ת���������ӷ���ʽΪ ��
��1���ҵ�����Ϊ ����ת���������ӷ���ʽΪ ��
��2��ʵ���ҿ�ͨ�����ַ����Ʊ��ף�����ͼ��ʾװ���ռ��ף����й�����ȡ���ռ���ʵ���������ȷ���� ��
�ټ����岻������ˮ���ռ�
��ʵ�����ñ�һ��ҩƷ������ȡ��
����ͼ��aΪ����ϡH2SO4����
|
��3����Aת��Ϊ�Ĺ����У�����ͼ��ʾ������
����λ��mol/L������2min��A��D��Ӧ���ɼ��ٶ�V���ף�= ��2minĩA��ת����Ϊ ��
��4����ҵ��ͨ���Լ�Ϊԭ���Ʊ�HNO3�����Ṥҵβ���е�NO��NO2�Ի�������Ⱦ������NaOH��Һ���գ�β����NO��NO2�����ʵ���֮��1��1��NaOH��Һ���յõ�һ�����Σ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
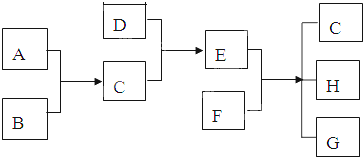
 ��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��?
��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��?
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ���¡���ѹ |
| ���� |
| ���¡���ѹ |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
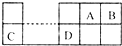 ��֪A��B��C��DΪ����������Ԫ�أ������λ�ù�ϵ��ͼ��C��B���γ����ӻ��� ��C3B2�����з�����ȷ���ǣ�������
��֪A��B��C��DΪ����������Ԫ�أ������λ�ù�ϵ��ͼ��C��B���γ����ӻ��� ��C3B2�����з�����ȷ���ǣ�������| A���縺�ԣ�C��A | B�����������ԣ�C��D | C���⻯���ȶ��ԣ�A��B | D��ԭ�Ӱ뾶��r��C����r��B�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com