
��15�֣� I ����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ���G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H���ܽ���A����G��HΪ���õ��ͻ����(ͼ�в��ֲ���û���г�)��
II��ͼ��ʾ����������G�Ĺ�ҵ���̣�
��1�����豸������Ϊ �����ҵĻ�ѧʽΪ M���ӵĽṹʽΪ ��
��2������X����Ҫ�ɷ�Ϊ ��
��3��д���豸���г����Ļ�ѧ��Ӧ ��
��4����ɫ����D��G��Һ��Ӧ�����ӷ���ʽΪ ��
��5����2.24 L����״���£�Eͨ��100mL2 mol/L A��ˮ��Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
��6��������ڹ���H���Ƶ�һ�ֽ��������⻯ѧ����ʽΪ �����ʱת��2.4mol���ӣ��Ƶý��� g��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����һ�и�����һ�������Ի�ѧ�Ծ� ���ͣ������
��12�֣���֪����ͼת����ϵ��ijЩת����ϵ�еIJ�������ȥ���ж�����ѧ��ѧ���������ʣ�����A��D��G�ǵ��ʣ����Ժ�ɫ����C��ij�����������Ҫ�ɷݣ�E��һ�ַ��������ᣬF�ǻ���H�Ǽ�������ˮ�ļ������塣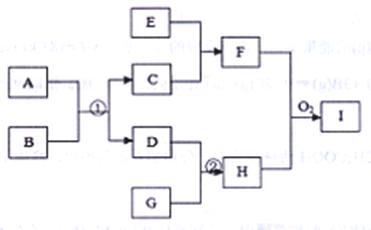
��ش��������⣺
��1��д���������ʵĻ�ѧʽ��A ��C ��I
��2��д����Ӧ�ٵĻ�ѧ����ʽ��
д�����ɻ����F�����ӷ���ʽ��
��3����һ���¶ȡ�ѹǿ���д������ڵ������½�l mol G��2,5 mol D����500mL�ܱ������С�����20min�ﵽƽ�⣬ƽ���H��Ũ��Ϊ2mol/L��
������G��ʾ20min�ڵ�ƽ����Ӧ����Ϊ��____
���¶��´˷�Ӧ��ƽ�ⳣ��K= ��D��ת����Ϊ
����������¶Ȳ��䣬����������ͬʱ����1��5 mol G��1 mol H��D��ת���ʽ� ������ߡ��������䡱���͡������ٴ�ƽ���H���������Ϊ____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ������ͨ���и����ڶ������Ͽ��ԣ����ۣ���ѧ���� ���ͣ������
��15�֣�I����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ���G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H���ܽ���A����G��HΪ���õ��ͻ����(ͼ�в��ֲ���û���г�)��
II��ͼ��ʾ����������G�Ĺ�ҵ���̣�
��1�����豸������Ϊ �����ҵĻ�ѧʽΪ M���ӵĽṹʽΪ ��
��2������X����Ҫ�ɷ�Ϊ ��
��3��д���豸���г����Ļ�ѧ��Ӧ ��
��4����ɫ����D��G��Һ��Ӧ�����ӷ���ʽΪ ��
��5����2.24 L����״���£�Eͨ��100 mL2 mol/L A��ˮ�� Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
��6��������ڹ���H���Ƶ�һ�ֽ��������⻯ѧ����ʽΪ �����ʱת��2.4mol���ӣ��Ƶý��� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�����ڶ������Ͽ��ԣ����ۣ���ѧ���� ���ͣ������
��15�֣� I ����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ���G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H���ܽ���A����G��HΪ���õ��ͻ����(ͼ�в��ֲ���û���г�)��

II��ͼ��ʾ����������G�Ĺ�ҵ���̣�

��1�����豸������Ϊ �����ҵĻ�ѧʽΪ M���ӵĽṹʽΪ ��
��2������X����Ҫ�ɷ�Ϊ ��
��3��д���豸���г����Ļ�ѧ��Ӧ ��
��4����ɫ����D��G��Һ��Ӧ�����ӷ���ʽΪ ��
��5����2.24 L����״���£�Eͨ��100 mL2 mol/L A��ˮ��Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
��6��������ڹ���H���Ƶ�һ�ֽ��������⻯ѧ����ʽΪ �����ʱת��2.4mol���ӣ��Ƶý��� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I ����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ���G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H���ܽ���A����G��HΪ���õ��ͻ����(ͼ�в��ֲ���û���г�)��

II��ͼ��ʾ����������G�Ĺ�ҵ���̣�

��1�����豸������Ϊ �����ҵĻ�ѧʽΪ M���ӵĽṹʽΪ ��
��2������X����Ҫ�ɷ�Ϊ ��
��3��д���豸���г����Ļ�ѧ��Ӧ ��
��4����ɫ����D��G��Һ��Ӧ�����ӷ���ʽΪ ��
��5����2.24 L����״���£�Eͨ��100 mL2 mol/L A��ˮ��Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
��6��������ڹ���H���Ƶ�һ�ֽ��������⻯ѧ����ʽΪ �����ʱת��2.4mol���ӣ��Ƶý��� g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com