
����Ŀ����֪25 ��ʱ����������ʵĵ���ƽ�ⳣ�����±���ʾ��
��ѧʽ | CH3COOH | H2CO3 | HClO | |
ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
�ش��������⣺
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1 mol��L-1��������Һ��
a. CH3COOH������ b. H2CO3 c. NaHCO3 d. HClO
pH��С���������˳����____(����ĸ)��
��2�������£�0.1 mol��L-1CH3COOH��Һ��ˮϡ�����У����б���ʽ����ֵ������____(����ĸ)��
A. c(H+) B.![]() C. c(H+)��c(OH-) D.
C. c(H+)��c(OH-) D.![]() E.
E.![]()
��3�������Ϊ100 mL��pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ����ͼ��ʾ����HX�ĵ���ƽ�ⳣ��____(����ڡ�����С�ڡ����ڡ�)CH3COOH�ĵ���ƽ�ⳣ����������____�������������pH����ͬ��CH3COOH��һԪ��HX�м���������п�����ɵ���������ͬ����������С��ϵΪ��CH3COOH____(����ڡ�����С�ڡ����ڡ�)HX
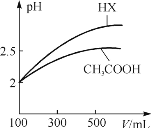
���𰸡�a<b<d<c BD ���� ��ˮϡ����ͬ����HX��pH�仯������ǿ������ƽ�ⳣ���� С��
��������
��1����ͬ���ʵ���Ũ�ȵ�������Һ���������ˮ��̶�Խ������Һ��pHԽ��
��2��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С��c��OH-������Kw���䣻
��3����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
��1���ɵ���ƽ�ⳣ���ж����Ե�ǿ��������Խǿ�����Ӧ�ε�ˮ��̶�Խ����Һ��pH��Խ���ɱ����е����ݿ�֪������CH3COOH��H2CO3��HClO��HCO3-�����������Խ����������ӵ�ˮ��̶�Խ����Һ�ļ���Խǿ������pH�ɴ�С��˳����a��d��c��b���ʴ�Ϊ��a��d��c��b��
��2��A.CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С����A��ѡ��
B.c��H+��/c��CH3COOH��=n��H+��/n��CH3COOH������ϡ�����б�ֵ���Bѡ��
C.ϡ���̣��ٽ����룬c��H+����С��c��OH-������c��H+��c��OH-��=Kw��Kw���䣬��C��ѡ��
D.ϡ���̣��ٽ����룬c��H+����С��c��OH-��������c��OH-��/c��H+�����Dѡ��
E.����ĵ���ƽ�ⳣ�����䣬��E��ѡ��
�ʴ�Ϊ��BD��
��3��ϡ����ͬ�ı�����pH�仯���������ǿ����ͼ��֪����ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ��������ǿ��HX�ĵ���ƽ�ⳣ���ȴ����pH��ͬ��һԪ�ᣬ��Խ�����Ũ��Խ����ͬ�����ͬpH�IJ�ͬһԪ��������п��Ӧ����Խ�����ɵ��������Խ������HX�����Աȴ��������ǿ���������ɵ���������ͬ����������HX��CH3COOH���ʴ�Ϊ�����ڣ���ˮϡ����ͬ����HX��pH�仯������ǿ������ƽ�ⳣ����С�ڡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��6.02��1023�����������ӵ����ʵ�����________mol����Ħ������Ϊ________��
��2���ڱ�״���£�0.01molij���������Ϊ0.44g�����������ܶ�Ϊ________g��L-1������С�������λ�������������Է�������Ϊ________��
��3����4g NaOH��������ˮ���250mL��Һ������Һ��NaOH�����ʵ���Ũ��Ϊ______��ȡ��10mL����Һ������10mL��Һ��ˮϡ�͵�100mL��ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ______��
��4����ͨ����һ�ֵ���ɫ���壬���������г�ɫ����ɫ������ɫ�ȶ��֣����Ƕ�����ĵ��ʣ�����ÿ����������ԭ�ӵĸ�����ͬ������Sx��ʾ�������������ⶨ�Ľ���ǣ���ɫ�������ܶ���ͬ״���������ܶȵ�64���������ķ���ʽΪ��______��
��5����18.0mol/L��Ũ����ϡ�ͳ�2.00mol/L��ϡ����100mL�����в�����������ҺŨ����ɵĺ�������ڡ�ƫ�͡�����______________
��δϴ��ϡ��Ũ�����С�ձ���
��ʹ�þ�����ˮϴ�Ӻ�δ�����С�ձ�ϡ��Ũ���
�ۼ�ˮʱ���������˿̶��ߣ��ֽ���������������������
������Һ�õ�����ƿ������ˮϴ�Ӻ�δ�����
�ݶ���ʱ���ӿ̶ȣ�
��ת����ƿҡ�Ⱥ�Һ�潵���̶����£��ټ�ˮ���̶ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
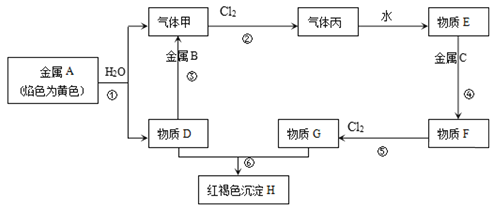
����Ŀ�����н�������A��B��C������ס����Լ�����D��E��F��G��H������֮����ת����ϵ��ͼ��ʾ��ͼ����Щ��Ӧ��������ͷ�Ӧ����û�б������
��ش��������⣺
��1��д���������ʵĻ�ѧʽ��B___����____��
��2��д�����з�Ӧ�����ӷ���ʽ��
��Ӧ��_____��
��Ӧ��_____��
��3����F����Һ�м���D��Һ�������������____���û�ѧ��Ӧ����ʽ���Ͳ����������ԭ��____��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ����������ȼ�ϵ����ˮ����ϵͳԭ����ͼ��ʾ��ͼ���л���ˮ���л������C6H10O5��ʾ�������й�˵����ȷ���ǣ�
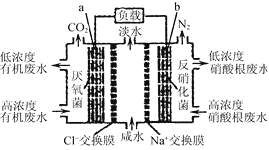
A. b�缫�Ϸ���������Ӧ
B. b�缫������Һ��pH����
C. a�缫��Ӧʽ��C6H10O5+24e����7H2O===6CO2����24H��
D. �м��ң�Na���������ң�Cl����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼa��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��ͼb�Ƿ�Ӧ�е�CO��NO��Ũ����ʱ��仯��ʾ��ͼ������ͼ��ش��������⣺
a 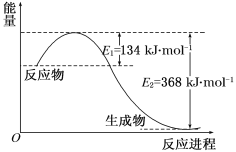 b
b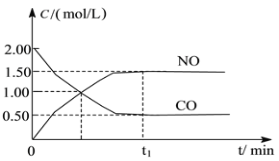
(1)д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ_______________��
(2)�ӷ�Ӧ��ʼ��ƽ�⣬��NO2Ũ�ȱ仯��ʾƽ����Ӧ����v(NO2)��__________��
(3)���¶��¸÷�Ӧ��ƽ�ⳣ��K=_________���¶Ƚ��ͣ�K________����������С�����䡱��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ҵ�л��������������ˮ�������������������������ŷš�
(1) ��ҵ����NaHSO3��ԭ���������������£������Է�ˮ�м���NaHSO3ʹCr2O72-��ԭ��ΪCr3+��Ȼ�������ʯ�ҵ��ڷ�ˮ��pH��ʹCr3+��ȫ������
д��NaHSO3��Cr2O72-��Ӧ�����ӷ���ʽ��_______________________��
(2) ��ˮ�и�Ԫ����Ũ�ȵIJⶨ�������£���һ������Cr2O72-��Cr3+�����Է�ˮ���м�������(NH4)2S2O8��Һ��Cr3+������Cr2O72-����г�ȥ������(NH4)2S2O8���ټ��������KI��Һ��Cr2O72-��I-��ȫ��Ӧ����Cr3+��I2���Ե���Ϊָʾ������Na2S2O3����Һ�ζ����յ㡣�ⶨ���������ʵ�ת����ϵ���£�Cr3+![]() Cr2O72-
Cr2O72-![]() I2
I2![]() S4O62-��
S4O62-��
���������������У�������в�������ⶨ�ĸ�Ԫ����Ũ�Ȼ�____(�ƫ����ƫС�����䡱)��
���Ե���Ϊָʾ������Na2S2O3����Һ�ζ����յ�ʱ������Ϊ________��
��ȷ��ȡ��Cr2O72-��Cr3+�����Է�ˮ��100.00 mL,�����������ⶨ��ˮ���и�Ԫ����Ũ��,����0.01000 mol��L-1Na2S2O3����Һ13.50 mL������÷�ˮ�и�Ԫ����Ũ��(��mg��L-1��ʾ)(д���������)__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ͭ�Ļ�����˵����ȷ����(����)
A. ��������ͭ������ǿ����ʵ��Ӧ���п���FeCl3��ʴCu����ӡˢ��·��
B. CuSO4��Һ��H2S��Һ��Ӧ�����ӷ���ʽΪ:Cu2++S2-![]() CuS��
CuS��
C. ��ϡ�����ȥͭ������ӷ���ʽΪCuO��2H��===Cu2����H2O
D. ��ѧ��Ӧ��CuO��CO![]() Cu��CO2��ʵ������Ϊ��ɫ�����ɺ�ɫ����
Cu��CO2��ʵ������Ϊ��ɫ�����ɺ�ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л�������Ľṹ��ʽ�ɽ�һ�����磺
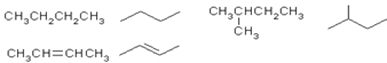
��1��������ĿҪ��ش��������⣺
�� ![]() ��ϵͳ�������Ը��л������������_____________________________________�����л���������������ѧ��Ӧ�Ļ�ѧ����ʽΪ���ýṹ��ʽ��д���� ________________________�����л���ѧ��Ӧ��������______________________��
��ϵͳ�������Ը��л������������_____________________________________�����л���������������ѧ��Ӧ�Ļ�ѧ����ʽΪ���ýṹ��ʽ��д���� ________________________�����л���ѧ��Ӧ��������______________________��
�� 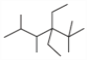 ��ϵͳ�������Ը��л������������__________________________________��
��ϵͳ�������Ը��л������������__________________________________��
�� ��-�¹�ϩ�Ľṹ��ʽΪ![]() ��һ���Ӹ��������������巢���ӳɷ�Ӧ�IJ��ֻ����λ���칹�������������_________�֡�
��һ���Ӹ��������������巢���ӳɷ�Ӧ�IJ��ֻ����λ���칹�������������_________�֡�
��2�����м����������ʣ�
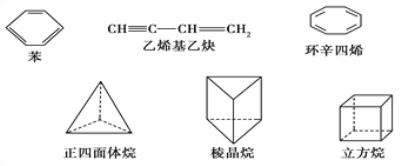
�� ����������Ķ���ȡ��������______�֣�������Ķ���ȡ��������______�֡�
�� ���ڱ��ͻ�����ϩ��˵����ȷ����________(����ĸ����)��
A������ʹ����KMnO4��Һ��ɫ
B��������H2�����ӳɷ�Ӧ���������ı��ͻ�����ϩ����H2�����ʵ���֮��Ϊ3:4
C������̼̼������̼̼˫������ṹ
D�����ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() Ϊ�����ӵ�����ֵ�������й�������ȷ����
Ϊ�����ӵ�����ֵ�������й�������ȷ����
A.��״���£�5.6LCO2�к��еĹ��õ��Ӷ���ĿΪ2NA
B.��״���£�22.4L���к��з��ӵ���ĿΪNA
C.1mol![]() ˮ�����ɵ�
ˮ�����ɵ�![]() ����������ΪNA
����������ΪNA
D.�ڷ�Ӧ![]() �У�ÿ����3mol
�У�ÿ����3mol![]() ת�Ƶĵ�����Ϊ
ת�Ƶĵ�����Ϊ![]()
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com