
�ҹ��ຣ������κ��̲��ŷḻ��ʳ����Դ��������֪�����ú�ˮ�к��и�Ũ�ȵ�Na����K����Mg2����Cl���ȡ������κ�ˮ�ɵõ�ijЩ���ʡ�����Ҫ��ҵ�������£�
������������̣��ش��������⣺
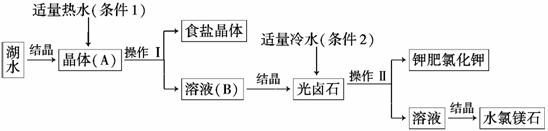
������������̣��ش��������⣺
(1)���ú�ˮ�õ�����(A)�ķ�����________�ᾧ��(����¡���������)��
(2)�����������Ϊ________���˲��������ڷ���______________��
(3)�������зֱ��������1������2��ò�ͬ���壬�� ���ݵ���������________��
���ݵ���������________��
a��Ħ��������b���ܽ�ȡ�c���ܽ��ԡ�d���۷е�
(4)����ط����Ƿ���Na����ʵ�鷽����________________��������Na�����ܹ۲쵽��������________________��
����õ��ߴ��ȵļطʣ����Բ��õ��ᴿ����Ϊ______________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��֤��һˮ�ϰ�(NH3��H2O)��������ʣ��ס��ҡ������˷ֱ�ѡ�������Լ�����ʵ�飺0.01 mol��L��1��ˮ��0.1 mol��L��1NH4Cl��Һ��NH4Cl���塢��̪�Լ���pH��ֽ������ˮ��
(1)����pH��ֽ���0.01 mol��L��1��ˮ��pHΪ10�����϶�һˮ�ϰ���������ʣ�����Ϊ��һ�����Ƿ���ȷ��(��ǡ���)________����˵�����ɣ�_________________________
________________________________________________________________________��
(2)��ȡ��10 mL 0.01 mol��L��1��ˮ����pH��ֽ�����pH��a��Ȼ��������ˮ����ϡ����1 000 mL������pH��ֽ�����pHΪb����Ҫȷ��һˮ�ϰ���������ʣ���a��bֵӦ����ʲô������________________________________________________________________________
(�õ�ʽ��ʽ��ʾ)��
(3)��ȡ��10 mL 0.0 1 mol��L��1��ˮ������2�η�̪��Һ���Էۺ�ɫ���ټ���NH4Cl������������ɫ��____________(����dz��)��
1 mol��L��1��ˮ������2�η�̪��Һ���Էۺ�ɫ���ټ���NH4Cl������������ɫ��____________(����dz��)��
(4)����������ṩ���Լ��������һ���Ⱥ����ּ��ķ���֤��һˮ�ϰ���������ʣ�________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ȼ�ͭ (CuCl2��2H2O)�к�FeCl2���ʡ�Ϊ�Ƶô����Ȼ�ͭ���壬���Ƚ����Ƴ�ˮ��Һ�� Ȼ��������ʾ������������ᴿ��

(1)����������x��Ŀ����
(2)���������������ʺϱ�ʵ�����
A��Cl2 B��KMnO4 C��NaClO D��H2SO4
(3)����y�ͳ���z�Ļ�ѧʽ�ֱ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪ��10 mLһ�����ʵ���Ũ�ȵ�����X��һ�����ʵ���Ũ�ȵ�NaOH��ҺY�ζ���ͼ������ͼ���Ƴ�X��Y������ ����Ũ�����±��ڸ����е�
����Ũ�����±��ڸ����е�
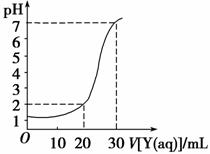
| A | B | C | D | |
| X/mol��L��1 | 0.12 | 0.04 | 0.03 | 0.09 |
| Y/mol��L��1 | 0.04 | 0.12 | 0.09 | 0.03 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������ʷ������(����)
����Ͳ������ͨ©�����۵ζ��ܡ�������ƿ���ݷ�Һ©��
��������ƿ
A���٢ۢݡ����������������� B���ڢܢ�
C���ڢݢ� D���ۢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵļ���ͷ��뷽����ȷ����(����)
A���ð�ˮ����Al3����Mg2����Ag��
B����Ba(NO3)2��Һ����Cl����SO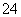 ��CO
��CO
C���ӵ�ˮ����ȡ���ʵ�ʱ����������ˮ�Ҵ�����CCl4
D��ʵ�������ᴿ��������������Ҵ����ɲ����ȼ���ʯ�ң����˺�������ķ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ���������̣�

�����������̻ش��������⣺
(1)��Ϣ��з�����Ӧ�Ļ�ѧ����ʽΪ________________________________��
(2)��Ϣ���ʹ�ñ�ˮ��Ŀ����________________________________��
(3)������Ͳ���������Ʒֱ���________��________��
������һ�������ڷ���________����(ѡ����)
a�������Һ�塡������b���������
c���������ܵ�Һ�� d�����ܵ�Һ��
(4)��Ϣ��м���Na2SO3��Ŀ����________��
(5)������������ӦΪ��ɫҺ�壬��ʵ�ʹ�ҵ�������Ƶõ�������(��ҵ������)���е����Ļ�ɫ�����Ǽ��ұ�ͬѧ����˼�ʵ�����̽������ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3����������֤���ü������õ��Լ�Ϊ________��������������ɹ۲쵽������Ϊ________����ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ________��������֤���ü������õ��Լ�Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ˮ����ͨ�����ȵ���ʱ����Ӧ�Ļ�ѧ����ʽΪ__________________________���ڷ�Ӧ����Ԫ�ػ��ϼ�______������__________��Ӧ�������ֳ���ǿ��____________������8.4g��������Ӧ��������������״���µ������ L��
��2����Ϊ�������������н�ǿ��____________��������Ȼ���еĴ����������____________̬���ڣ�ֻ�м������������õĽ�����____________����____________̬���ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y������C��H ��O����Ԫ����ɵ���Է�������С��240�ķ����廯�����֪��
��O����Ԫ����ɵ���Է�������С��240�ķ����廯�����֪��
��.������X��Y������Ԫ��C��H��O�������Ⱦ�Ϊ9��1��3��
��.X��Y��Ϊͬ���칹�壬��X��Y�����Է������л�ѧ��Ӧ��
X��H2O A1��B1(
A1��B1( C5H12O)
C5H12O)
Y��H2O A2��B2
A2��B2
B1��B2��Ϊͬϵ�B1��B2����Է��������Ĺ�ϵΪMr(B1)��Mr(B2)��42��
(1)X��Y�ķ���ʽ��____________��
(2)A1�ķ���ʽ��________��A1�ܹ������Ļ�ѧ��Ӧ��________(�����)��
��������Ӧ����������Ӧ���������Ȼ�����Һ������ɫ��Ӧ������ȥ��Ӧ��������ˮ����ȡ����Ӧ��������ˮ�����ӳɷ�Ӧ
(3)A2��Ũ���Ṳ�ȿ��Է�����ȥ��Ӧ��Ҳ������ͭ�������������±��������������������������Ƶ�������ͭ����Һ�������ɵ��л��ﱽ���ϵ�һ�ȴ���ֻ��һ�֡�
��Y�Ľṹ��ʽΪ______________________________________________��
��A2��Ũ���Ṳ�ȷ�����ȥ��Ӧ�Ļ�ѧ����ʽΪ___________________________________
_______________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com