



| “߻ƼĮ |
| “߻ƼĮ |
| “߻ƼĮ |
| “߻ƼĮ |
 £®ŌŁ½įŗĻ¶ŌÓ¦ÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ£¬ŅŌ¼°Ē°ŗó×Ŗ»Æ¹ŲĻµ½ā“šøĆĢā£®
£®ŌŁ½įŗĻ¶ŌÓ¦ÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ£¬ŅŌ¼°Ē°ŗó×Ŗ»Æ¹ŲĻµ½ā“šøĆĢā£® £¬¹Ź“š°øĪŖ£ŗH2O2£»CO£»
£¬¹Ź“š°øĪŖ£ŗH2O2£»CO£» £»
£»| “߻ƼĮ |
| “߻ƼĮ |
| “߻ƼĮ |
| “߻ƼĮ |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ



| “߻ƼĮ |
| “߻ƼĮ |
| “߻ƼĮ |
| “߻ƼĮ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©ĖüĆĒµÄŌŖĖŲ·ūŗÅŹĒA__________£¬B__________£¬C__________”£
£Ø2£©ĖüĆĒµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄ»ÆѧŹ½ĪŖA__________£¬B__________£¬C__________”£
£Ø3£©ĖüĆĒµÄ×īøß¼ŪŃõ»ÆĪļµÄ»ÆѧŹ½ĪŖA__________£¬B__________£¬C__________£¬ĘäÖŠĖįŠŌ×īĒæµÄŹĒ__________£¬×īČõµÄŹĒ__________”£
£Ø4£©AµÄµ„ÖŹŌŚøßĪĀĻĀÓėBµÄ×īøß¼ŪŃõ»ÆĪļ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________£¬“Ė·“Ó¦µÄÓĆĶ¾ŹĒ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
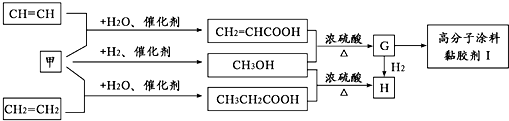
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2005-2006ѧğ½ĖÕŹ”ŠģÖŻŹŠøßŅ»£ØĻĀ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
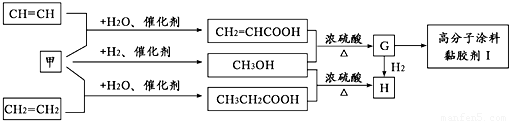
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com