
������Ͳ��ȡ5.4 mLŨH2SO4������ע��װ��Լ50mL������ˮ���ձ��ﲢ�ò��������衣
����Լ30 mL����ˮ����3��ϴ���ձ��Ͳ���������ÿ��ϴ��Һ����������ƿ�
�۽�ϡ�Ͳ���ȴ���H2SO4С�ĵص�������ƿ�
�ܼ��100 mL����ƿ�ڲ��Ƿ�ᷢ����©��
�ݽ�����ˮֱ�Ӽ�������ƿ����Һ��ӽ��̶���0.5��l crrl����
�ǽ�ƿ�����ߵ���ҡ����Һ��
���ý�ͷ�ι�������ƿ����ε�������ˮ����Һ����͵�ǡ�úͿ̶������С�
�ݴ���д��
(1)��ȷ�IJ���˳����(����ű�ʾ)______________��
(2)����(1)����ʱ��Ӧѡ�����������е�(�����)________��
a.10 mL��Ͳ b.50 mL��Ͳ c.500 mL��Ͳ
d.1 000 mL��Ͳ
�����װ��ŨH2SO4����Ͳ����ʱ�����ӣ����Ƶ�ϡH2SO4��Ũ�Ƚ�________ (��ƫ�ߡ���ƫ�͡�����Ӱ�족)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.��98%��Ũ��������100 g 10%��ϡ������Һʱ����ʹ����Ͳ����õ�����ƿ
B.����������������л��е�NaNO3����
C.���÷�Һ©�����Ҷ�����ˮ�Ļ��Һ�����
D.ij��ɫ��Һ�м������������ɫ��ζ���壬����Һ��һ����![]()
E.SO2����ϩ����Ȳͨ����ˮ���ܱ�������ʹ��ˮ��ɫ
F.�ü�ʽ�ζ�����ȡ20.00 mL 0.100 0 mol��L-1��KMnO4��Һ
H.����ѧ������ͭ������ᾧˮ�����ⶨ����ʵ���У���������������Ҫ�Ĵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ�ɶ����и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
ij�о���ѧϰС���������²�����̽��NH3�Ļ�ԭ�ԣ�����ʵ��װ������ͼ��
����1��NH3��ǿ��ԭ�����ܽ�ijЩ���������ﻹԭΪ�������ʻ�ͼ�̬��������磺
2NH3 + 3CuO  3Cu + N2 +3H2O
3Cu + N2 +3H2O
����2��Cu+��������Һ�в��ȶ����ɷ�������������ԭ��Ӧ����Cu2O����ɫ��������Cu2+��Cu��
Cu2O + 2H+ ="=" Cu2+ + Cu +H2O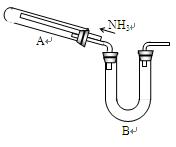
��ش��������⣺
(1)Ϊ֤��NH3��ԭCuO�ķ�Ӧ����ˮ���ɣ�B��Ӧ������Լ��� ��
(2)���۲쵽 ��������������A�еķ�Ӧ�Ѿ���ɣ�
(3)��С�����������Ϊ1�U4��ϡ�������Լ������鷴Ӧ�Ƿ���Cu2O�������ɡ�����98%��Ũ��������1�U4��ϡ���ᣬ����IJ����������˽�ͷ�ι���� ��
(4)��֤����ԭ�����к���Cu2O�IJ����������� ��
(5)��д��A������Cu2O�Ļ�ѧ����ʽ ��
(6)���ö����ķ����ⶨ�÷�Ӧ�Ƿ�����Cu2O��������ȷ�ķ�����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ̩���и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йػ�ѧʵ��������ȷ����
A������ƿ���ζ��ܡ���Һ©��ʹ��ǰ���������Ƿ�©ˮ
B����98%��Ũ��������100g10%��ϡ����ʱ����ʹ����Ͳ����õ�����ƿ
C�����Ũ����ʱ��ʹ�ô���������ɫ����ƿ
D��ȡ�û�ѧҩƷ��ʵ�飬ʣ��ҩƷ������ͬһ��Һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
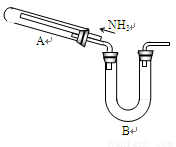
ij�о���ѧϰС���������²�����̽��NH3�Ļ�ԭ�ԣ�����ʵ��װ������ͼ��
����1��NH3��ǿ��ԭ�����ܽ�ijЩ���������ﻹԭΪ�������ʻ�ͼ�̬��������磺
2NH3 + 3CuO  3Cu +
N2 +3H2O
3Cu +
N2 +3H2O
����2��Cu+��������Һ�в��ȶ����ɷ�������������ԭ��Ӧ����Cu2O����ɫ��������Cu2+��Cu��
Cu2O + 2H+ == Cu2+ + Cu +H2O
��ش��������⣺
(1)Ϊ֤��NH3 ��ԭCuO�ķ�Ӧ����ˮ���ɣ�B��Ӧ������Լ��� ��
(2)���۲쵽 ��������������A�еķ�Ӧ�Ѿ���ɣ�
(3)��С�����������Ϊ1�U4��ϡ�������Լ������鷴Ӧ�Ƿ���Cu2O�������ɡ�����98%��Ũ��������1�U4��ϡ���ᣬ����IJ����������˽�ͷ�ι���� ��
(4)��֤����ԭ�����к���Cu2O�IJ����������� ��
(5)��д��A������Cu2O�Ļ�ѧ����ʽ ��
(6)���ö����ķ����ⶨ�÷�Ӧ�Ƿ�����Cu2O��������ȷ�ķ�����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com