
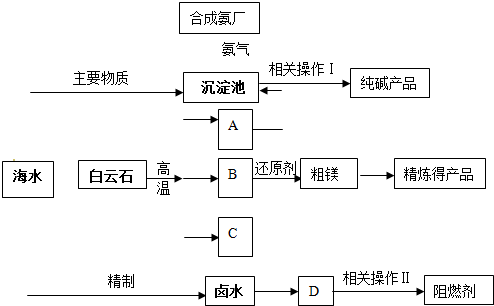
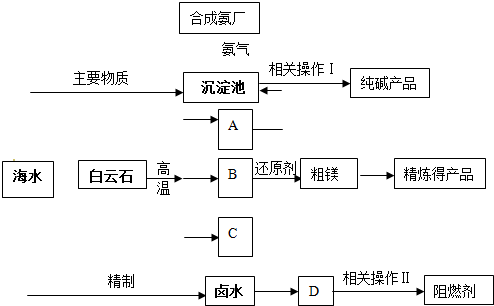
ij�����������������þ����ȼ��������ʯ��CaCO3��MgCO3��Ϊԭ��ұ��þ���������������ɵ��������ڻ�ԭ¯�о�1200���ù�����ԭ����þ�����������ϼ۲��䣩��þ����������Ϊ��þ��ͬʱ�Ժ�ˮΪԭ���Ƽ������ȼ���������������£�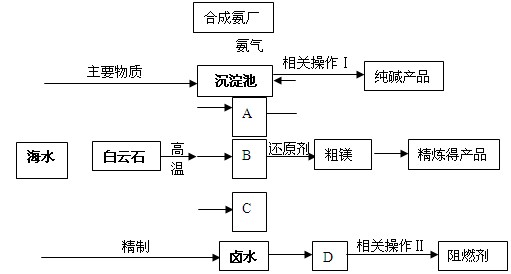
��1���ֱ�д��A��B��C�Ļ�ѧʽ �� �� ���������з�Ӧ�Ļ�ѧ����ʽ�� ��
��2��������ԭ����þ����ʱѡ���ѹ��1200�棬�Է���ѡ�����ַ�Ӧ������ԭ��
��
��3������±ˮ�е�MgCl2������ʯ���鷴Ӧ���ɼ�ʽ�Ȼ�þ[Mg(OH)Cl]��������ʱ��Һ����Ҫ�����ǣ�д��ѧʽ�� ��
��4��������������ز�����������ˡ� �� ��
��5����֪��Mg(OH)2(s)��MgO(s)+H2O(g)��81.5kJ��Al(OH)3(s)��0.5Al2O3(s)+1.5 H2O(g)��87.7kJ
��Mg(OH)2������ȼ���ã���ԭ���� ��
�ڵ�����Mg(OH)2��Al(OH)3��ȣ���ȼЧ���Ϻõ��� ��
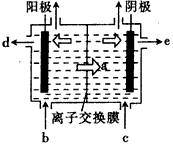
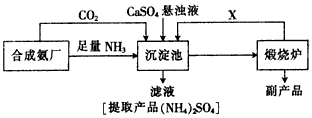
��6��ij������������ͼ��ʾ���������������þ����ȼ�����Է������������ŵ�
��
������12�֣�
��1��CO2 MgO CaO ��3�֣���1�֣���CO2+NH3+NaCl+H2O��NaHCO3��+ NH4Cl��1�֣�
��2����ѹ������þ���������ɼ����룻1200��ֻ��þΪ���壬�����ʺ�ƽ��2���Ƕȶ�������þ�����ɣ��������ɣ���2�֣���
��3��CaCl2��1�֣� ��4��ϴ�ӡ����գ�����ȣ���2�֣�
��5����Mg(OH)2���ȷֽ�ʱ���մ�������ʹ�����¶��½���ͬʱ���ɵ����¡��ȶ��Ժõ�MgO�������ڿ�ȼ����棬������ȼ���á���1�֣� ��Mg(OH)2��1�֣�
��6��ԭ���ü�����ԭ�������ʸߡ��м��������Ч��ѭ�����á���Ʒ�ṹ�Ķ�������1�֣����1�㼴�ɣ�
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
 A��ʳ�εı�����Һ
A��ʳ�εı�����Һ ����Һ
����Һ ����
���� ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����������������þ����ȼ��������ʯ��CaCO3��MgCO3��Ϊԭ��ұ��þ���������������ɵ��������ڻ�ԭ¯�о�1200���ù�����ԭ����þ�����������ϼ۲��䣩��þ����������Ϊ��þ��ͬʱ�Ժ�ˮΪԭ���Ƽ������ȼ���������������£�

![]()
|
|
|
|
|
|
|
|
��1���ֱ�д��A��B��C�Ļ�ѧʽ �� �� ���������з�Ӧ�Ļ�ѧ����ʽ�� ��
��2��������ԭ����þ����ʱѡ���ѹ��1200�棬�Է���ѡ�����ַ�Ӧ������ԭ��
��
��3������±ˮ�е�MgCl2������ʯ���鷴Ӧ���ɼ�ʽ�Ȼ�þ[Mg(OH)Cl]��������ʱ��Һ����Ҫ�����ǣ�д��ѧʽ�� ��
��4��������������ز�����������ˡ� �� ��
��5����֪��Mg(OH)2(s)��MgO(s)+H2O(g)��81.5kJ��Al(OH)3(s)��0.5Al2O3(s)+1.5 H2O(g)��87.7kJ
��Mg(OH)2������ȼ���ã���ԭ���� ��
�ڵ�����Mg(OH)2��Al(OH)3��ȣ���ȼЧ���Ϻõ��� ��
��6��ij������������ͼ��ʾ���������������þ����ȼ�����Է������������ŵ�
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com