
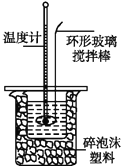
 ����������д�����з�Ӧ���Ȼ�ѧ����ʽ��
����������д�����з�Ӧ���Ȼ�ѧ����ʽ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����V1��V2��c1=c2������Һ��pH��7 |
| B���������Һ��pH=7����c1V1��c2V2 |
| C����c1=c2�����Һ��c��NH4+���Tc��Cl-������V1��V2 |
| D���������Һ��pH��7������Һ��c��NH4+����c��OH-����c��Cl-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.1mol/LCH3COOH��Һ��ˮϡ�ͣ���Һ��c��OH-������ | ||
B����NaOH�Ͱ�ˮ��Һ��ϡ��һ�������ߵ�OH-Ũ�Ⱦ����ٵ�ԭ����
| ||
| C����ͬŨ�ȵ�HCl��CH3COOH������Һ��c��H+����ͬ | ||
| D����HA��HB��Ϊ���ᣬ����HA��HB������ͬ�����£���Һ��pH��СΪNaA��NaB |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 VA��ĵ����ס��飨As����Ԫ�صĻ������ڿ��к���������������Ҫ��;����ش��������⣮
VA��ĵ����ס��飨As����Ԫ�صĻ������ڿ��к���������������Ҫ��;����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
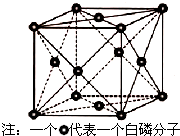
 �Ĵ�ʢ���屶�ӣ����屶��Ϊԭ�Ͽ����Ƶû�����A��A�Ľṹ��ʽ��ͼ��ʾ����ش��������⣺
�Ĵ�ʢ���屶�ӣ����屶��Ϊԭ�Ͽ����Ƶû�����A��A�Ľṹ��ʽ��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ͨ�������У�NH3+H+=NH4+ |
| B������ͭм�ŷ�ϡ�����У�3Cu+8H++2NO3-=3Cu2++2NO��+4H2O |
| C������SO2ͨ��������ռ���Һ�У�SO2+2OH-=SO32-+H2O |
| D������������ĩ�е��백ˮ��Al��OH��3+OH-=AlO2-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��12.5g |
| B��13.2g |
| C��19.7g |
| D��24.4g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com