
| Ąė×Ó | Na+ | Mg2+ | Cl- | SO42- |
| ÅضČ/£Øg•L-1£© | 63.7 | 28.8 | 144.6 | 46.4 |
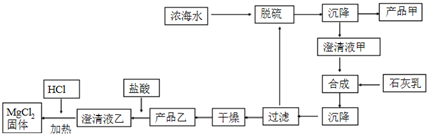
·ÖĪö £Ø1£©“µ³öBr2£¬ÓĆSO2ĪüŹÕ£¬·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉĮņĖįŗĶHBr£¬Br2+Na2CO3+H2O”śNaBr+NaBrO3+NaHCO3ÖŠBrÓÉ0½µµĶĪŖ-1£¬ÓÉ0ÉżøßĪŖ+5¼Ū£¬æÉÖŖĪüŹÕ3moläå×ŖŅĘ5molµē×Ó£»
£Ø2£©ÓÉĮ÷³ĢæÉÖŖ£¬ÅØŗ£Ė®ÖŠĄūÓĆøĘĄė×Ó½«ĮņĖįøłĄė×Ó×Ŗ»ÆĪŖ³Įµķ£¬µĆµ½²śĘ·¼×ĪŖĮņĖįøĘ£»ĀĖŅŗ¼×ŌŚŗĻ³É²½ÖčÖŠ¼ÓČėŹÆ»ŅČ飬½«Ć¾Ąė×Ó×Ŗ»ÆĪŖ³Įµķ£¬¹żĀĖ”¢øÉŌļŗóµĆµ½µÄ²śĘ·ŅŅĪŖĒāŃõ»ÆĆ¾³Įµķ£»¼ĘĖć1LČÜŅŗÖŠMg2+µÄÖŹĮ棬øł¾ŻMg2+”«Mg£ØOH£©2¼ĘĖćĒāŃõ»ÆĆ¾µÄÖŹĮ森
½ā“š ½ā£ŗ£Ø1£©“µ³öBr2£¬ÓĆSO2ĪüŹÕ£¬·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉĮņĖįŗĶHBr£¬ĘäÄæµÄŹĒŹ¹Br2ø»¼Æ£»Br2+Na2CO3+H2O”śNaBr+NaBrO3+NaHCO3ÖŠBrÓÉ0½µµĶĪŖ-1£¬ÓÉ0ÉżøßĪŖ+5¼Ū£¬æÉÖŖĪüŹÕ3moläå×ŖŅĘ5molµē×Ó£¬ŌņĪüŹÕ0.15mol Br2Ź±£¬×ŖŅʵĵē×ÓĪŖ0.15mol”Į$\frac{5}{3}$=0.25mol£¬
¹Ź“š°øĪŖ£ŗŹ¹Br2ø»¼Æ£»0.25£»
£Ø2£©ÓÉĮ÷³ĢæÉÖŖ£¬ÅØŗ£Ė®ÖŠĄūÓĆøĘĄė×Ó½«ĮņĖįøłĄė×Ó×Ŗ»ÆĪŖ³Įµķ£¬µĆµ½²śĘ·¼×ĪŖĮņĖįøĘ£»ĀĖŅŗ¼×ŌŚŗĻ³É²½ÖčÖŠ¼ÓČėŹÆ»ŅČ飬½«Ć¾Ąė×Ó×Ŗ»ÆĪŖ³Įµķ£¬¹żĀĖ”¢øÉŌļŗóµĆµ½µÄ²śĘ·ŅŅĪŖĒāŃõ»ÆĆ¾³Įµķ
¢ŁøĆ¹¤ŅÕ¹ż³ĢÖŠ£¬ĶŃĮņ½×¶ĪÖ÷ŅŖµÄĄė×Ó·½³ĢŹ½ĪŖCa2++SO42-=CaSO4”ż£¬¼ÓČėŹÆ»ŅČ鏱Ėł·¢ÉśµÄĄė×Ó·½³ĢŹ½ŹĒMg2++Ca£ØOH£©2=Mg£ØOH£©2”ż+Ca2+£¬
¹Ź“š°øĪŖ£ŗCa2++SO42-=CaSO4”ż£»Mg2++Ca£ØOH£©2=Mg£ØOH£©2”ż+Ca2+£»
¢ŚÓÉÉĻŹö·ÖĪöæÉÖŖ£¬²śĘ·ŅŅĪŖMg£ØOH£©2£¬
ČÜŅŗÖŠm£ØMg2+£©=1L”Į28.8g/L=28.8g£¬
Mg2+”«Mg£ØOH£©2
24g 58g
28.8g m[Mg£ØOH£©2]
m[Mg£ØOH£©2]=28.8g”Į$\frac{58g}{24g}$=69.6g£¬
¹Ź“š°øĪŖ£ŗMg£ØOH£©2£»69.9g£®
µćĘĄ ±¾Ģāæ¼²éŗ£Ė®×ŹŌ“æŖ·¢ĄūÓĆ£¬ĪŖøßĘµæ¼µć£¬Éę¼°Ńõ»Æ»¹Ō·“Ó¦”¢Ąė×Ó·“Ó¦¼°Į÷³Ģ·ÖĪö£¬×¢ÖŲ»ł“”ÖŖŹ¶µÄ×ŪŗĻŌĖÓĆ£¬²ąÖŲ·ÖĪöÓėÓ¦ÓĆÄÜĮ¦µÄ漲飬ĢāÄæÄѶČÖŠµČ£®


 æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø
æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | äå±½ÖŠ»ģÓŠä壬¼ÓČėKIČÜŅŗ£¬Õńµ“£¬ÓĆĘūÓĶŻĶČ”³öäå | |
| B£® | ŅŅĶéÖŠ»ģÓŠŅŅĻ©£¬ĶØH2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦£¬Ź¹ŅŅĻ©×Ŗ»ÆĪŖŅŅĶé | |
| C£® | Ļõ»ł±½ÖŠ»ģÓŠÅØH2SO4ŗĶÅØHNO3£¬½«Ęäµ¹ČėNaOHČÜŅŗÖŠ£¬¾²ÖĆ£¬·ÖŅŗ | |
| D£® | ŅŅĻ©ÖŠ»ģÓŠCO2ŗĶSO2£¬½«ĘäĶعżŹ¢ÓŠNaOHČÜŅŗµÄĻ“ĘųĘæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | µķ·Ū£ØĀČ»ÆÄĘ£©ÉųĪö | |
| B£® | Ó²Ö¬ĖįÄĘ£ØøŹÓĶČÜŅŗ£©ŃĪĪö”¢¹żĀĖ | |
| C£® | Ė®£Ø¼¦µ°Ē壩ÕōĮó | |
| D£® | ÕįĢĒ£ØĘĻĢŃĢĒ£©ÓėŅų°±ČÜŅŗ»ģŗĻĖ®Ō”¼ÓČČ£¬¹żĀĖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2NaCl£ØČŪČŚ£©$\frac{\underline{\;µē½ā\;}}{\;}$2Na+Cl2”ü | B£® | MgO+H2$\frac{\underline{\;\;”÷\;\;}}{\;}$Mg+H2O | ||
| C£® | Fe2O3+3CO$\frac{\underline{\;øßĪĀ\;}}{\;}$2Fe+3CO2 | D£® | 2Ag2O$\frac{\underline{\;\;”÷\;\;}}{\;}$4Ag+O2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ”°Ė®µĪŹÆ“©”±Ö÷ŅŖŹĒČܽāĮĖCO2µÄÓźĖ®ÓėCaCO3³¤ĘŚ×÷ÓĆÉś³ÉĮĖæÉČÜŠŌCa£ØHCO3£©2µÄŌµ¹Ź | |
| B£® | ³¤ĘŚŹ¢·ÅNaOHČÜŅŗµÄŹŌ¼ĮĘæ²»ŅדņæŖ£¬ŹĒŅņĪŖNaOHÓėĘæÖŠµÄCO2·“Ó¦µ¼ÖĀĘæÄŚĘųĢå¼õÉŁŠĪ³ÉøŗŃ¹µÄŌµ¹Ź | |
| C£® | ŃĻøńµŲ½²£¬ŹµŃéŹŅŹ¹ÓĆ”±ĶØ·ē³÷”±·ĄĪŪČ¾ŹĒ²»øŗŌšČĪµÄ£¬ŅņĪŖŹµŃé²śÉśµÄÓŠŗ¦ĘųĢåƻӊµĆµ½×Ŗ»Æ»ņĪüŹÕ | |
| D£® | ”°Óźŗó²Źŗē”±Óė”°ŗ£ŹŠņ×Ā„”±¶¼ŹĒ×ŌČ»½ēµÄ¹āѧĻÖĻó£¬Ņ²Óė½ŗĢåÖŖŹ¶ÓŠ¹Ų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £¬Ęä×īøßŃõ»ÆĪļĖ®»ÆĪļÓė¢ŽŗÅŌŖĖŲ×īøß¼ŪŃõ»ÆĪļĖ®»ÆĪļ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl£ØOH£©3+OH-=[Al£ØOH£©4]-_£®
£¬Ęä×īøßŃõ»ÆĪļĖ®»ÆĪļÓė¢ŽŗÅŌŖĖŲ×īøß¼ŪŃõ»ÆĪļĖ®»ÆĪļ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl£ØOH£©3+OH-=[Al£ØOH£©4]-_£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĖįŠŌ£ŗH4SiO4£¼H3PO4£¼H2SO4£¼HClO4 | B£® | ¼īŠŌ£ŗCa£ØOH£©2£¾Mg£ØOH£©2£¾Al£ØOH£©3 | ||
| C£® | Ēā»ÆĪļµÄĪČ¶ØŠŌ£ŗSiH4£¾H2S£¾H2O£¾HF | D£® | Ō×Ó°ė¾¶£ŗF£¼O£¼S£¼Na |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·ÅµēŹ±£¬LiMn2O4·¢ÉśŃõ»Æ·“Ó¦£¬µē³ŲÄŚ²æLi+ĻņÕż¼«ŅĘ¶Æ | |
| B£® | ·ÅµēŹ±£¬Õż¼«·“Ó¦ĪŖ£ŗLi++LiMn2O4+e-ØTLi2Mn2O4 | |
| C£® | “×ĖįæÉÓĆ×÷ļ®Ąė×Óµē³ŲµÄµē½āÖŹ | |
| D£® | ³äµēŹ±£¬ļ®µÄĢ¼²ÄĮĻĪŖŃō¼«ĒŅ·“Ó¦ĪŖ£ŗLi++e-ØTLi |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com