



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���ڢۢ� |
| C���ڢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ȩ�������õ�����һԪ�� |
| B����ȩ����ȩ����ȩ��û��ͬ���칹�� |
| C����2%��ϡ��ˮ��μ���2%��AgNO3��Һ�У�������ǡ���ܽ�Ϊֹ�����Ƶ�������Һ |
| D����2%��NaOH��Һ4��6�Σ�����2mL 10%��CuSO4��Һ���Ƶ�Cu��OH��2����Һ����������ȩ�����Լ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
��
 ��
��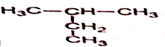
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
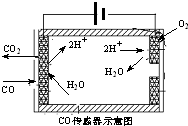 �������ִ������а�����Խ��Խ��Ҫ�Ľ�ɫ������β����̼�⻯����������Pһ����̼�ȣ������Ļ�����ȾԽ��Խ���ԣ������������ŷ��ѳ�Ϊ���д�����Ⱦ����Ҫ��Դ��
�������ִ������а�����Խ��Խ��Ҫ�Ľ�ɫ������β����̼�⻯����������Pһ����̼�ȣ������Ļ�����ȾԽ��Խ���ԣ������������ŷ��ѳ�Ϊ���д�����Ⱦ����Ҫ��Դ��| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������Ȼ��ܵ����ʣ��ں��в�ͬ��Ŀ�Ľᾧˮ����ʱ���ֲ�ͬ����ɫ������ʼ���ˮ���У��Ƴɱ�ɫˮ�� |
| B���մɾ��п�����������ʴ�����¡���Ե���׳��͵��ŵ� |
| C������û�й̶����۵㣬������״̬ʱ�����Ա����Ƴ��κ���״����Ʒ |
| D�����ά����Ҫ�ɷ־��ǹ赥�ʣ����Ƿdz��õ�ͨ�Ų��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com