
¢ŁÕż¼«£ŗµē¼«·“Ó¦Ź½ĪŖ£ŗ4H2O+2O2+8e-====8OH-
¢Śøŗ¼«£ŗµē¼«·“Ó¦Ź½ĪŖ£ŗ4OH--4e-====2H2O+O2”ü
¢Ūøŗ¼«£ŗµē¼«·“Ó¦Ź½ĪŖ£ŗCH4+10OH--8e-====![]() +7H2O
+7H2O
¢ÜÕż¼«£ŗµē¼«·“Ó¦Ź½ĪŖ£ŗ4H++4e-====2H2”ü
A.¢Ł¢Ś B.¢Ū¢Ü C.¢Ł¢Ū D.¢Ś¢Ü

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 £Ø2011?Ģ«Ō¶žÄ££©ĻÖŅŃČ·ČĻ£¬CO”¢SO2ŗĶNOxµÄÅÅ·ÅŹĒŌģ³É“óĘųĪŪČ¾µÄÖŲŅŖŌŅņ£®
£Ø2011?Ģ«Ō¶žÄ££©ĻÖŅŃČ·ČĻ£¬CO”¢SO2ŗĶNOxµÄÅÅ·ÅŹĒŌģ³É“óĘųĪŪČ¾µÄÖŲŅŖŌŅņ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(8·Ö)µ„ÖŹA”¢B”¢CŌŚ³£ĪĀĻĀ¾łĪŖĘųĢ¬£¬·Ö±šÓɼה¢ŅŅ”¢±ūČżÖÖ¶ĢÖÜĘŚŌŖĖŲ×é³É£»ŅŃÖŖ±ūŌŖĖŲµÄŌ×Ó½į¹¹ÖŠ“ĪĶā²ćµē×ÓŹż±Č×īĶā²ćµē×ÓŹż¶ą1£¬»ÆŗĻĪļDŌŚ³£ĪĀĻĀ³ŹŅŗĢ¬£¬GŹĒ³£¼ūµÄ½šŹōµ„ÖŹ£¬HŹĒ³£¼ūµÄĄ¶É«³Įµķ£¬ø÷ĪļÖŹ¼äµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾(·“Ó¦Ģõ¼ž¶ąŹżŅŃĀŌČ„)£ŗ
Ēė»Ų“š£ŗ
(1)·“Ó¦¢ŽµÄĄė×Ó·½³ĢŹ½ŹĒ_______________________________________________”£
(2)¼×”¢ŅŅĮ½ŌŖĖŲ³żŠĪ³É»ÆŗĻĪļDĶā£¬»¹æÉŠĪ³ÉŅ»ÖÖŗ¬ÓŠ4øöŌ×ÓŗĖŗĶ18øöµē×ӵĻÆŗĻĪļ£¬øĆ»ÆŗĻĪļµÄµē×ÓŹ½ŹĒ________”£
(3)ŌŚ·“Ó¦¢ŁÖŠ£¬ŅŃÖŖ1 g BĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬DŹ±£¬·Å³ö142.9 kJµÄČČĮ棬Ōņ±ķŹ¾BµÄČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ŹĒ_______________________________________________
________________________________________________________________________ӣ
(4)·“Ó¦¢ŁŅ²æÉŅŌÉč¼Ę³ÉŌµē³Ų×°ÖĆ½ųŠŠ£¬µ±ÓĆ²¬×÷µē¼«£¬ÓĆKOHČÜŅŗ×÷µē½āÖŹČÜŅŗŹ±£¬øŗ¼«µÄµē¼«·“Ó¦Ź½ŹĒ________________________________________________________
________________________________________________________________________ӣ
(5)·“Ó¦¢Ū¢ÜŌŚĶس£ĒéæöĻĀ²»ÄÜ×Ō·¢½ųŠŠ£¬æņĶ¼ÖŠµÄĢõ¼žaŹĒ________£¬ŌŚŹ¹·“Ó¦¢Ū¢ÜÄܹ»·¢ÉśµÄ×°ÖĆÖŠ£¬ÓŠŅ»øöµē¼«²ÄĮĻ±ŲŠėĻąĶ¬£¬Š“³öøƵē¼«·“Ó¦Ź½________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ½Ī÷Ź”øßČżÉĻѧʌµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¾Ż±ØµĄ£¬Ņ»¶ØĢõ¼žĻĀÓɶžŃõ»ÆĢ¼ŗĶĒāĘųŗĻ³ÉŅŅ“¼ŅŃ³ÉĪŖĻÖŹµ”£
ŅŃÖŖ£ŗ¢ŁCH3CH2OH(l) +3 O2 (g) = 2CO2(g) +3H2O(l) ”÷H=£1366.8 kJ/ mol
¢Ś2H2 (g) + O2 (g) = 2H2O(l) ”÷H= -571.6 kJ/ mol
£Ø1£©Š“³öÓÉCO2 ŗĶH2 ·“Ó¦ŗĻ³É CH3CH2OH (l) ŗĶH2O(l)µÄČČ»Æѧ·½³ĢŹ½ ”£
£Ø2£©¼īŠŌŅŅ“¼Č¼ĮĻµē³ŲŅד¢“ę£¬Ņ×ĶĘ¹ć£¬¶Ō»·¾³ĪŪČ¾Š”£¬¾ßÓŠ·Ē³£¹ćĄ«µÄ·¢Õ¹Ē°¾°”£øĆČ¼ĮĻµē³ŲÖŠ£¬Ź¹ÓĆ²¬×÷µē¼«£¬KOHČÜŅŗ×öµē½āÖŹČÜŅŗ”£ĒėŠ“³öøĆČ¼ĮĻµē³Ųøŗ¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ”£
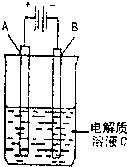
£Ø3£©ÓĆŅŅ“¼Č¼ĮĻµē³Ųµē½ā400 mL ±„ŗĶŹ³ŃĪĖ®×°ÖĆæɼņµ„±ķŹ¾ČēĻĀĶ¼£ŗ

øĆ×°ÖĆÖŠ·¢Éśµē½ā·“Ó¦µÄ·½³ĢŹ½ĪŖ £»ŌŚĢś°ōø½½ü¹Ū²ģµ½µÄĻÖĻóŹĒ £»µ±Ņõ¼«²śÉś448 mLĘųĢå£ØĢå»żŌŚ±ź×¼×“æöĻĀ²āµĆ£©Ź±£¬Ķ£Ö¹µē½ā£¬½«µē½āŗóµÄČÜŅŗ»ģŗĻ¾łŌČ£¬ČÜŅŗµÄpHĪŖ ”££Ø²»æ¼ĀĒĘųĢåµÄČܽā¼°ČÜŅŗĢå»żµÄ±ä»Æ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com