
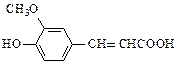 ��2�֣�
��2�֣� ��4Cu(OH)2 ������
��4Cu(OH)2 ������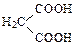 ��2Cu2O��4H2O��3�֣�
��2Cu2O��4H2O��3�֣�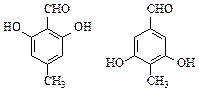 ��2�֣�
��2�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
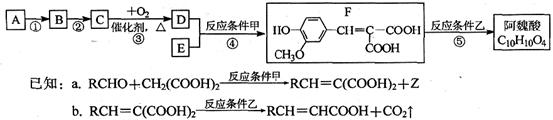
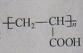 �ĵ��壬��A�к��еĹ������� ��д���ƣ���B�Ľṹ��ʽ�� ��
�ĵ��壬��A�к��еĹ������� ��д���ƣ���B�Ľṹ��ʽ�� �� b.��ʹ���Ը��������Һ��ɫ
b.��ʹ���Ը��������Һ��ɫ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��M����ϡ������Һ��Ӧ���ɴ������� | |
| B��Imol M�ڴ��������������������5mol H2��Ӧ | |
| C��1mol M����2mol NaOH����ˮ�ⷴӦ | D��M��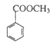 ��Ϊͬϵ�� ��Ϊͬϵ�� |
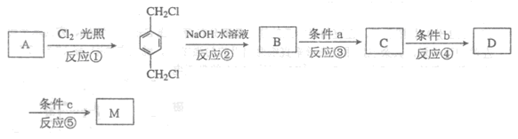
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
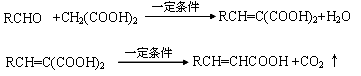

 �����ʵ���C�ֱ���������Na��Ũ��ˮ��NaOH��NaHCO3��Ӧʱ����Na��Br2��NaOH��NaHCO3�����ʵ���֮����________________
�����ʵ���C�ֱ���������Na��Ũ��ˮ��NaOH��NaHCO3��Ӧʱ����Na��Br2��NaOH��NaHCO3�����ʵ���֮����________________ �ð�κ����һ�����������ɿ������Եĸ߷��ӻ�����ķ���ʽ��
�ð�κ����һ�����������ɿ������Եĸ߷��ӻ�����ķ���ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
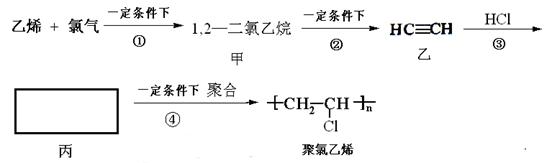
 ��
���鿴�𰸺ͽ���>>
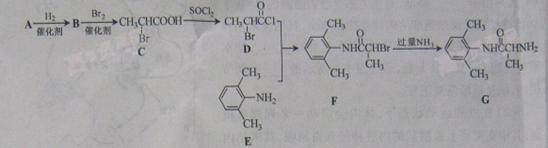
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
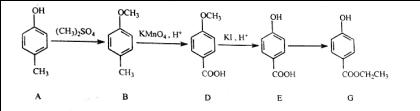
 B��D
B��D E������������ .
E�����Ĺ������� . G�Ļ�ѧ����ʽΪ .
G�Ļ�ѧ����ʽΪ .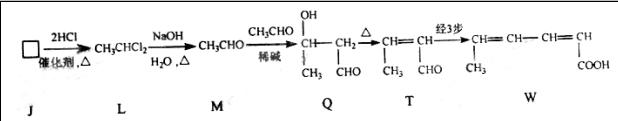
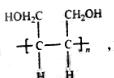 ������
��Ϊ����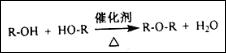 ��������������Ʊ�;��Ϊ������ �� .���Ӧ���ͣ�
��������������Ʊ�;��Ϊ������ �� .���Ӧ���ͣ�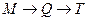 ��ԭ������T�Ʊ�W�ķ�Ӧ����Ϊ
��ԭ������T�Ʊ�W�ķ�Ӧ����Ϊ�鿴�𰸺ͽ���>>
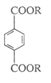
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���ױ�����Q�����������ֻ�ѧ������ͬ����ԭ�ӣ����������ʾ�������ԭ�Ӹ�����Ϊ1��3����ش��������⣺
���ױ�����Q�����������ֻ�ѧ������ͬ����ԭ�ӣ����������ʾ�������ԭ�Ӹ�����Ϊ1��3����ش��������⣺
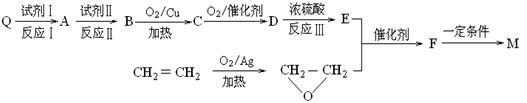

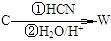 ����д�������������������ʵ�һ�ֽṹ��ʽ ��
����д�������������������ʵ�һ�ֽṹ��ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
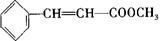 )�����ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��
)�����ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��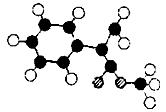

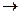 G�ķ�Ӧ������ ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
G�ķ�Ӧ������ ���÷�Ӧ�Ļ�ѧ����ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com