
��06���������Ǧ�����ǵ��͵Ŀɳ��͵�أ���������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��
Pb+PbO2+4H++2SO42-![]() 2PbSO4+2H2O
2PbSO4+2H2O
��ش��������⣨�������⡢����������ԭ����
(1)�ŵ�ʱ�������ĵ缫��Ӧʽ��___________________________
____________________________________________________�����Һ��H2SO4��Ũ�Ƚ���________________�������·ͨ��1 mol����ʱ�������ϸ��������������______________g��
(2)����ȫ�ŵ�ľ�PbO2��Pbʱ��������27ͼ���ӣ����һ��ʱ�������A�缫������________________________��B�缫������_______________________����ʱǦ���ص��������ļ��Խ�_______________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06���������Ϊ��ȥ�����е�Ca2+��Mg2+��Fe3+��SO42�D �Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
![]()
 (1)�ж�BaCl2�ѹ����ķ�����___________________________________
(1)�ж�BaCl2�ѹ����ķ�����___________________________________
__________________________________________________________________________________________________________________��
(2)�ڢܲ��У���ص����ӷ���ʽ��______________________________
________________________________________________________��
(3)�����������pHֵ�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
___________________________________________________________________________________________________________________��
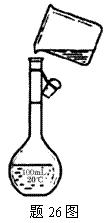
(4)Ϊ���龫�δ��ȣ�������150 mL.0.2 mol/L NaCl(����)��Һ����26ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����_________________
_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06���������Ϊ��ȥ�����е�Ca2+��Mg2+��Fe3+��SO42�D �Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
![]()
 (1)�ж�BaCl2�ѹ����ķ�����___________________________________
(1)�ж�BaCl2�ѹ����ķ�����___________________________________
__________________________________________________________________________________________________________________��
(2)�ڢܲ��У���ص����ӷ���ʽ��______________________________
________________________________________________________��
(3)�����������pHֵ�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
___________________________________________________________________________________________________________________��
(4)Ϊ���龫�δ��ȣ�������150 mL.0.2 mol/L NaCl(����)��Һ����26ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����_________________
_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06���������X��Y��Z��W��ԭ��������������Ķ�����Ԫ�أ��һ���ͬ�壻����ֻ������Ϊ������Xԭ�ӵ������������������������ȣ�X��W��Y��Z������ԭ�ӵ�����������֮�;�Ϊ9������Y��W������Ũ��NaOH��Һ��Ӧ����ش��������⣺
(1)Y��Z��W��ԭ�Ӱ뾶��С�����˳����___________________________________��
(2)ZW2�ĵ���ʽ��_____________�����ڳ����³�Һ̬���γɾ���ʱ������_________���塣
(3)��ҵ��������Y��ԭ����___________________________________________________(�û�ѧ����ʽ��ʾ)��
(4)X��Y��ѧ�������ƣ���X��Ũ��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________________________________________________��
(5)0.1 mol�ĵ���W��50 mL 1.5 mol/L��FeBr2��Һ��Ӧ����������Fe2+ ��Br�D �����ʵ���֮����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06���������X��Y��Z��W��ԭ��������������Ķ�����Ԫ�أ��һ���ͬ�壻����ֻ������Ϊ������Xԭ�ӵ������������������������ȣ�X��W��Y��Z������ԭ�ӵ�����������֮�;�Ϊ9������Y��W������Ũ��NaOH��Һ��Ӧ����ش��������⣺
(1)Y��Z��W��ԭ�Ӱ뾶��С�����˳����___________________________________��
(2)ZW2�ĵ���ʽ��_____________�����ڳ����³�Һ̬���γɾ���ʱ������_________���塣
(3)��ҵ��������Y��ԭ����___________________________________________________(�û�ѧ����ʽ��ʾ)��
(4)X��Y��ѧ�������ƣ���X��Ũ��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________________________________________________��
(5)0.1 mol�ĵ���W��50 mL 1.5 mol/L��FeBr2��Һ��Ӧ����������Fe2+ ��Br�D �����ʵ���֮����____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com