�⣺��1�������ƺ�ˮ��Ӧ��������������Ӱ���������������Ӱ����Ʒ�й������Ƶ������ⶨ������ȡ����ʱ��������е������ƹ���û�б�Ҫ��ȥ��
�ʴ�Ϊ��û�У���Ϊ�����ƺ�ˮ��Ӧ��������������Ӱ�������������
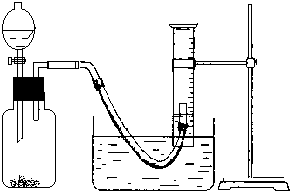
��2��ʵ��ͨ���ռ������ⶨ���ɵ������������������Ʒ�й������Ƶ���������������������ƵĴ��ȣ���ʵ�鿪ʼӦ����װ�õ������ԣ�
�ʴ�Ϊ������װ�õ������ԣ�
��3����Ӧ�ų��������ȣ���������������������ʣ�Ӧ��ʹ����ָ������£��ٽ��ж�ȡ���������������ʱӦ������Ͳ�߶�ʹ��Ͳ���������ѹǿ��ȣ���ʹ����Һ����ƽ����Ӧ����ȴ���ٵ�ƽ��Ͳ����Һ�棬����������ȷ˳���Ǣڢ٢ۣ�
�ʴ�Ϊ���ڢ٢ۣ�
��4����״���£�ʵ���еõ������������B L����������Ƶ�����Ϊm����
2Na
2O
2+2H
2O=4NaOH+H
2����
2��78g 22.4L
m BL
��m=
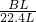
��2��78g=
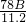
g
�ʹ������ƵĴ���Ϊ

��100%=
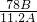
��100%��
�ʴ�Ϊ��
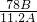
��100%��
��������1�������ƺ�ˮ��Ӧ��������������Ӱ���������������Ӱ����Ʒ�й������Ƶ������ⶨ��
��2��ʵ��ͨ���ռ������ⶨ���ɵ������������������Ʒ�й������Ƶ���������������������ƵĴ��ȣ���ʵ�鿪ʼӦ����װ�õ������ԣ�
��3����Ӧ�ų��������ȣ���������������������ʣ�Ӧ��ʹ����ָ������£��ٽ��ж�ȡ���������������ʱӦ������Ͳ�߶�ʹ��Ͳ���������ѹǿ��ȣ���ʹ����Һ����ƽ��
��4������������ˮ��Ӧ2Na
2O
2+2H
2O=4NaOH+H
2�����������ɵ�������������÷���ʽ����������Ƶ������������ô��ȵĶ������������ƵĴ��ȣ�
���������⿼��ʵ��ԭ�������⡢ʵ��������������ʺ����ⶨ����ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ��

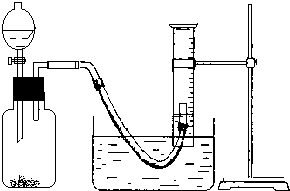 ��Ag���������ƵĹ������ƹ���������ȡ�������ⶨ���й������ƵĴ��ȣ��ɹ�ѡ��ķ�Ӧ�ﻹ�У�����ˮ�����Իش��������⣺
��Ag���������ƵĹ������ƹ���������ȡ�������ⶨ���й������ƵĴ��ȣ��ɹ�ѡ��ķ�Ӧ�ﻹ�У�����ˮ�����Իش��������⣺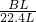 ��2��78g=
��2��78g=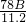 g
g ��100%=
��100%=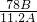 ��100%��
��100%��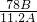 ��100%��
��100%��

 ����������ϵ�д�
����������ϵ�д�
 ��Ag���������ƵĹ������ƹ���������ȡ�������ⶨ���й������ƵĴ��ȣ��ɹ�ѡ��ķ�Ӧ�ﻹ�У�����ˮ�����Իش��������⣺
��Ag���������ƵĹ������ƹ���������ȡ�������ⶨ���й������ƵĴ��ȣ��ɹ�ѡ��ķ�Ӧ�ﻹ�У�����ˮ�����Իش��������⣺