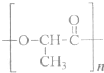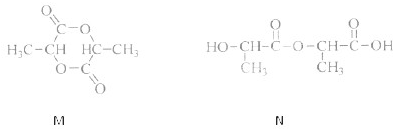����Ϊѡ���⣮����A��B���⣮ѡѧ����ѧ�����ģ��Ŀ�����A�⣮ѡѧ���� ����ѧ������ģ��Ŀ�����B�⣮ÿλ����ֻ��ѡ��1�⣮�����ⶼ��������A��Ʒ֣�
A������ѧ�����
��1�����������������ʣ���a��ʳ�� ��b��ʳ�� ��c��ƻ��֭ ��d�������� ��e����ù�أ��밴����Ҫ����գ�����ţ���
��������C����
c
c
����ֱ�ӽ���ѪҺ������������
d
d
��Ӧ����㷺�Ŀ�����֮һ����
e
e
��������Ϊ��ζ�����ֿ�Ԥ����ð����
b
b
��ʳ�ù��������Ѫѹ���ߡ����������
a
a
��
��2�����λ�����Ⱦ��������̬�����ѳ�Ϊȫ��Ĺ�ʶ��
�ٿ�����������ĸ���ָ����Է�ӳ�����ؿ��������������������������ҹ����������������
C
C
������ĸ����
a��CO
2 b��N
2 c��NO
2������Ӧ�����ռ������¡���ɫ��Ⱦ������������Ӧ����������
a
a
������ĸ����־������Ͳ�ڣ�
�۹�ҵ��ˮ�账����������ŷţ����з�ˮ�����ķ�����������
a
a
������ĸ����
a�����кͷ���ȥ��ˮ�е���
b���û�������ȥ��ˮ�е��ؽ�������
c����������ȥ��ˮ�е�������
��3�����������������������Ҫ���ʻ���������ѧ�Dz��Ͽ�ѧ��չ�Ļ���������д���пո�
�������е��մɡ�ˮ���
����
����
���ڴ�ͳ�������β��ϣ�������������Ҫ��ʯ��ʯ��ԭ�ϵ���
�մ�
�մ�
��
����ԭ�ӷ�Ӧ���е��Թ㷺Ӧ�õ��ƼغϽ��ڳ����³�Һ̬��˵���Ͻ���۵����ɷֽ������۵�
��
��
����ߡ��͡�����
�����жԽ�����Ʒ��ȡ�ķ�����������ȷ����
C
C
������ţ���
A���ڵ��ߵ��������һ�����ϲ� B�������г���Ȧ�϶���һ�������
C���ں��ֵ���������Ϻ���ͭ��
��4��ij����Ʒ��װ��ӡ��������ϣ���ѡ���⡢ʳ�Ρ��������ơ��������ƣ��������ڵ�ζ������
ʳ��
ʳ��
�����ڷ�ɫ������
��������
��������
�����ڷ���������
��������
��������
��
B�����л���ѧ����������Уѧ��ͳһ��ѡ����
��1����3�֣����ݽṹ���л�����з��࣬�����ڶ������ʵ����գ�
�������л������ڷ���������
c
c
������ĸ����
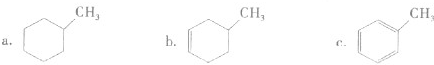
�������л������ڷ������
a
a
������ĸ����
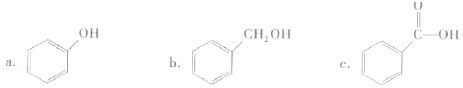
��������������������
b
b
������ĸ����
a����֬ b����ά�� c��������
��2��������X�Ľṹ��ʽΪ��
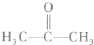
��X�ĺ˴Ź�������ͼ��H�˴Ź�����ͼ������
1
1
�����շ壮
��X��һ��ͬ���칹��Y�ܷ���������Ӧ����д��Y�Ľṹ��ʽ��
CH3-CH2-CHO
CH3-CH2-CHO
��
��X����һ��ͬ���칹��z�Ľṹ��ʽΪH
2C�TCHCH
2OH�����Z��Br
2�����ӳɷ�Ӧ�Ļ�ѧ����ʽ��
H2C�TCHCH2OH+Br2=H2CBrCHBrCH2OH
H2C�TCHCH2OH+Br2=H2CBrCHBrCH2OH
��
��3��2010���Ϻ�����������������ɫ����������ܻ��������������洦�ɼ�����һ���Բͺ�����һ������ɽ���ĸ߷��Ӻϳɲ����Ƶã���ṹ��ʽ���£�
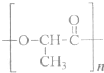
�ٸø߷��Ӳ�������һ�ֵ���ͨ��
����
����
��Ӧ���Ӧ���ͣ��Ʊ����ɣ��䵥��Ľṹ��ʽΪ
��
���������ӵĸõ��巢����Ӧ���ȿ��ܵõ���״������M��Ҳ���ܵõ���״������N����ṹ��ʽ���£�
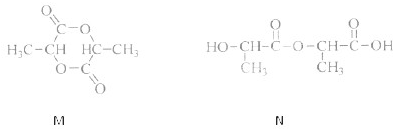
�������ַ�������M��N������һ��
ȡ���ֲ���ֱ����Na2CO3��Һ�����������ɵ���N�����������ɵ���M
ȡ���ֲ���ֱ����Na2CO3��Һ�����������ɵ���N�����������ɵ���M
����������
ȡ���ֲ���ֱ����˴Ź������ף�1H�˴Ź�����ͼ�ϳ���2������M������6������N
ȡ���ֲ���ֱ����˴Ź������ף�1H�˴Ź�����ͼ�ϳ���2������M������6������N
��




 ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�