
NH3��һϵ�з�Ӧ���Եõ�HNO3��NH4NO3������ͼ��ʾ��
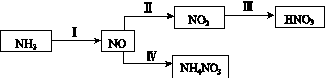
(1)���У�NH3��O2�ڴ��������·�Ӧ���仯ѧ����ʽ��_________________________��
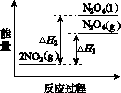
(2)����2NO(g)��O2(g)  2NO2(g)��������������
2NO2(g)��������������
ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ(p1��p2)�����¶ȱ仯������(��ͼ)��
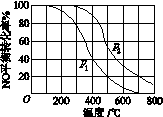
�ٱȽ�p1��p2�Ĵ�С��ϵ��________��
�����¶����ߣ��÷�Ӧƽ�ⳣ���仯��������________��
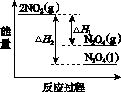
(3)���У������¶ȣ���NO2(g)ת��ΪN2O4(l)�����Ʊ�Ũ���ᡣ
����֪��2NO2(g)  N2O4(g)����H1 2NO2(g)
N2O4(g)����H1 2NO2(g) N2O4(l)����H2
N2O4(l)����H2
���������仯ʾ��ͼ�У���ȷ����(ѡ����ĸ)________��
 ��
��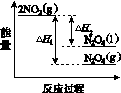
������A������������������B C
��N2O4��O2��H2O���ϵĻ�ѧ����ʽ��________________________________________��
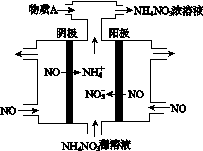
(4)���У����NO�Ʊ�NH4NO3���乤��ԭ����ͼ��ʾ��Ϊʹ������ȫ��ת��ΪNH4NO3���貹��A��A��________��˵�����ɣ�________________________________________��
(1)4NH3��5O2 4NO��6H2O
4NO��6H2O
(2)��p1��p2���ڼ�С
(3)��A����2N2O4��O2��2H2O===4HNO3
(4)NH3�����ݷ�Ӧ��8NO��7H2O 3NH4NO3��2HNO3����������HNO3��
3NH4NO3��2HNO3����������HNO3��
[����] (1)���Ĵ������ķ�Ӧ����ʽΪ
4NH3��5O2 4NO��6H2O��
4NO��6H2O��
(2)����2NO(g)��O2(g)  2NO2(g)��֪�÷�ӦΪ���������С�ķ�Ӧ���¶���ͬ������ѹǿ��ƽ�������ƶ���NO��ƽ��ת����������ͼʾ�����꺬�壬�ж�p1<p2�����ٿ�ͬһѹǿ�ߣ��¶����ߣ�NO��ƽ��ת���ʽ��ͣ�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�ⳣ����С��
2NO2(g)��֪�÷�ӦΪ���������С�ķ�Ӧ���¶���ͬ������ѹǿ��ƽ�������ƶ���NO��ƽ��ת����������ͼʾ�����꺬�壬�ж�p1<p2�����ٿ�ͬһѹǿ�ߣ��¶����ߣ�NO��ƽ��ת���ʽ��ͣ�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�ⳣ����С��
(3)�ٽ����¶ȣ�NO2(g)ת��ΪN2O4(l)����H2<0����Ӧ����������������������C���� N2O4(g)ת��ΪN2O4(l)��Ҫ�ų�����������NO2(g)ת��ΪN2O4(g)��NO2(g)ת��ΪN2O4(l)�ų��������٣�B����������ȷ��ͼʾΪA����N2O4��O2��H2O��Ӧ��������ķ�Ӧ����ʽΪ2N2O4�� O2��2H2O===4HNO3��
(4)���ݹ���ԭ��װ��ͼ������ȷ������ΪNOʧȥ����ת��ΪNO ������NOת��ΪNH
������NOת��ΪNH �����ݵ缫��Ӧ��д�缫��ӦʽΪ��
�����ݵ缫��Ӧ��д�缫��ӦʽΪ��
������NO��3e����2H2O===NO ��4H��
��4H��
������NO��5e���� 6H��===NH �� H2O
�� H2O
Ȼ����ݵ�ʧ�����غ㣬������������ʵ�����笠��������ʵ����࣬������Ҫ����Һ�м��������ΪNH3(��8NO��7H2O 3NH4NO3��2HNO3)��
3NH4NO3��2HNO3)��


 ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������������ϡ���ᷴӦ���ų�NO���ʵ���������(����)
A��FeO B��Fe2O3 C. FeSO4 D��Fe3O4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ػ�ѧ֪ʶ�����жϣ����н��۴������(����)
A��ij���ȷ�Ӧ���Է����У���˸÷�Ӧ��������Ӧ
B��NH4Fˮ��Һ�к���HF�����NH4F��Һ���ܴ���ڲ����Լ�ƿ��
C����ȼ����Ҫ�Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�ˮ���ᄃ�壬��˿ɴ����ں���
D������Ӧ��Ũ�ȿɼӿ췴Ӧ���ʣ������Ũ����������Ӧ����������H2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£�10 mL 0.40 mol/L H2O2��Һ�������ֽ⡣��ͬʱ�̲������O2�����(������Ϊ��״��)���±���
| t/min | 0 | 2 | 4 | 6 | 8 | 10 |
| V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ����(��Һ����仯���Բ���)(����)
A��0��6 min��ƽ����Ӧ���ʣ�
v(H2O2)��3.3��10��2mol��L��1��min��1
B��0��6 min��ƽ����Ӧ���ʣ�
v(H2O2)<3.3��10��2mol��L��1��min��1
C����Ӧ��6 minʱ��c(H2O2)��0.30 mol/L
D����Ӧ��6 minʱ��H2O2�ֽ���50%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������OH�������·���ˮ�ⷴӦ��
O2NC6H4COOC2H5��OH�� O2NC6H4COO����C2H5OH
O2NC6H4COO����C2H5OH
���ַ�Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.050 mol��L��1��15 ��ʱ���O2NC6H4COOC2H5��ת��������ʱ��仯�����������ʾ���ش��������⣺
| t/s | 0 | 120 | 180 | 240 | 330 | 530 | 600 | 700 | 800 |
| ��/% | 0 | 33.0 | 41.8 | 48.8 | 58.0 | 69.0 | 70.4 | 71.0 | 71.0 |
(1)��ʽ����÷�Ӧ��120��180 s��180��240 s �����ƽ����Ӧ����________��________���Ƚ����ߴ�С�ɵó��Ľ�����____________________��
(2)��ʽ����15 ��ʱ�÷�Ӧ��ƽ�ⳣ��________��
(3)Ϊ���O2NC6H4COOC2H5��ƽ��ת���ʣ������ʵ����Ʒ�Ӧ�¶��⣬���ɲ�ȡ�Ĵ�ʩ��________(Ҫ��д������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ú̿ȼ�չ����л��ͷų�������SO2�������ƻ���̬����������һ����������������Ԫ����CaSO4����ʽ�̶����Ӷ�����SO2���ŷš�����ú̿ȼ�չ����в�����CO�ֻ���CaSO4������ѧ��Ӧ����������Ч�ʡ���ط�Ӧ���Ȼ�ѧ����ʽ���£�
CaSO4(s)��CO(g) CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaSO4(s)��4CO(g) CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
��ش��������⣺
(1)��Ӧ���ܹ��Է����еķ�Ӧ������________��
(2)�����������ķ�Ӧ����ʾƽ�ⳣ��Kpʱ���������(B)��ƽ��ѹǿp(B)������������ʵ�����Ũ��c(B)����Ӧ���Kp��________(�ñ���ʽ��ʾ)��
(3)����ij�¶��£���Ӧ�������(v1 )���ڷ�Ӧ�������(v2 )�������з�Ӧ���������仯ʾ��ͼ��ȷ����________��
(4)ͨ����ⷴӦ��ϵ������Ũ�ȵı仯���жϷ�Ӧ��͢��Ƿ�ͬʱ������������____________________________________________________________________��
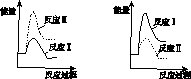
�������������� ��A������������B
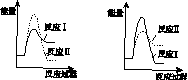
�������������� ��C������������D
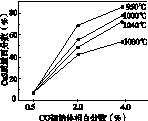
(5)ͼ(a)Ϊʵ���ò�ͬ�¶��·�Ӧ��ϵ��CO��ʼ����ٷ�����ƽ��ʱ���������CaS�����ٷ����Ĺ�ϵ���ߡ��÷�Ӧ��ϵ��SO2�������Ĵ�ʩ��________��
A����÷�Ӧ��ϵ��Ͷ��ʯ��ʯ
B���ں��ʵ��¶�������ƽϵ͵ķ�Ӧ�¶�
C�����CO�ij�ʼ����ٷ���
D����߷�Ӧ��ϵ���¶�
(6)���º��������£����跴Ӧ��͢�ͬʱ��������v1>v2������ͼ(b)������Ӧ��ϵ��c(SO2)��ʱ��t�仯��������ͼ��
 ��
��
����������(a)������������������������(b)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����仯�����������������ϵ���С�
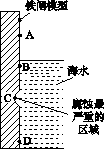
(1)��ͼ��ʵ�����о���ˮ����բ��ͬ��λ��ʴ���������ʾ��ͼ��
�ٸõ绯��ʴ��Ϊ________��
��ͼ��A��B��C��D�ĸ�������������������________(����ĸ)��
(2)�÷���Ƥ��ȡ����(Fe2O3)�IJ�������ʾ��ͼ���£�
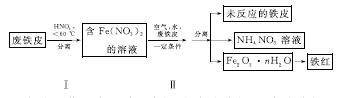
�ٲ�������¶ȹ��ߣ�����������ֽ⡣����ֽ�Ļ�ѧ����ʽΪ______________________________��
�ڲ�����з�����Ӧ��4Fe(NO3)2��O2��(2n��4)H2O===2Fe2O3��nH2O��8HNO3����Ӧ������HNO3�ֽ�����Ƥ�е���ת��ΪFe(NO3)2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
���������������У������֡���ɫ��ѧ��˼�����______(��дһ��)��
(3)��֪t ��ʱ����ӦFeO(s)��CO(g)Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25��
��t ��ʱ����Ӧ�ﵽƽ��ʱn(CO)��n(CO2)��________��
������1 L�ܱ������м���0.02 mol FeO(s)����ͨ��x mol CO, t ��ʱ��Ӧ�ﵽƽ�⡣��ʱFeO(s)ת����Ϊ50%����x��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ȼ�����Ƶ���H2��CO��Ϊ��Ҫ��ɵĹ�ҵԭ�Ϻϳ�������ӦΪCH4(g)��H2O(g)
 CO(g)��3H2(g)��
CO(g)��3H2(g)��
(1)������ˮ������Ӧ����������Ԫ����____________�������ɱ�״����35.84 L�ϳ���ʱת�Ƶ��ӵ����ʵ�����________��
(2)��2 mol CH4��5 mol H2O(g)ͨ���ݻ�Ϊ100 L�ķ�Ӧ�ң�CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼK235��ʾ��
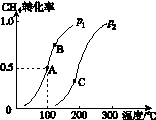
ͼK235
�����ﵽA�������ʱ��Ϊ5 min����v(H2)��________________________________________________________________________��
100 ��ʱƽ�ⳣ��K��____________________��
��ͼ�е�p1______p2(�<����>������)��A��B��C�����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��________________________________________________________________________��
(3)�ϳ������ںϳɰ���ʱ���ȥCO��������ӦCO(g)��H2O(g) 
 CO2(g)��H2(g)����H<0�����д�ʩ����ʹ
CO2(g)��H2(g)����H<0�����д�ʩ����ʹ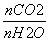 �������________(ѡ����)��
�������________(ѡ����)��
A�������¶�
B�����º����³���He(g)
C����H2����ϵ�з���
D����ͨ��һ������ˮ����
����̼�����Һ�������ɵ�CO2��������pH��10��̼�����Һ����ˮ�����OH�������ʵ���Ũ��Ϊ________________________________________________________________________��
�����£�0.1 mol��L��1 KHCO3��Һ��pH>8������Һ��c(H2CO3)________c(CO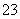 )(�>����������<��)��
)(�>����������<��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й���������ȡ������Ʒ��
�ش��������⣺
(1)���иĽ����Ż���ˮ�ۺ����ù��յ�������������е���________(�����)��
���û�������ȡ��ˮ
����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ��
�ܸĽ��ء��塢þ�ȵ���ȡ����
(2)���á���������������Ũ��ˮ����Br2�����ô������ա������������Ҫ��Ӧ��Br2��Na2CO3��H2O��NaBr��NaBrO3��NaHCO3������1 mol Br2ʱ��ת�Ƶĵ�����Ϊ________mol��
(3)��ˮ��þ��һ�ι�����������ͼ��

Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na�� | Mg2�� | Cl�� | SO |
| Ũ��/(g��L��1) | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ______________________________����Ʒ2�Ļ�ѧʽΪ__________��1 LŨ��ˮ���ɵõ���Ʒ2������Ϊ________g��
(4)����ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ________________________�����ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ��____________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com