
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ14��������Ϊ7��X��������NH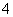 ������ͬ�����ӡ�������Ŀ��W��Y����������ܵ���������γɣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��
������ͬ�����ӡ�������Ŀ��W��Y����������ܵ���������γɣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��
(1)Y�����ڱ��е�λ����__________________��
(2)�õ���ʽ����X��W���γɻ�����X3W��ԭ��____________________��
(3)X3W��ˮ���ͷų�ʹ��̪��Һ��������A����ѧ����ʽ��____________________________________��
(4)�ö��Ե缫��⻯����XZ��Һ�������ͷų�����B����Ӧ�����ӷ���ʽ��__________________��
 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
I���Ʊ�Na2S2O3•5H2O
��Ӧԭ����Na2SO3��aq��+S��s��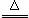 Na2S2O3(aq)
Na2S2O3(aq)
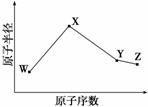
ʵ�鲽�裺
�ٳ�ȡ15g Na2SO3����Բ����ƿ�У��ټ���80ml����ˮ����ȡ5g��ϸ����ۣ���3ml �Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ�ã���ͼ��ʾ�����ּӳ�װ����ȥ����ˮԡ���ȣ���60���ӡ�
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3•5H2O�������ˣ�ϴ�ӣ�����õ���Ʒ��
�ش����⣺
��1������ڷ�Ӧǰ���Ҵ���ʪ��Ŀ���� ��
��2������a�������� ���������� ��
��3����Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ��������� �������Ƿ���ڸ����ʵķ����� ��
��4����ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ���ʽ��ʾ��ԭ��
��
II.�ⶨ��Ʒ����
ȷ��ȡWg��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.1000 mol•L‾1��ı���Һ�ζ���
��Ӧԭ��Ϊ��2S2O32‾+I2=S4O62-+2I‾
 ��5���ζ����յ�ʱ����Һ��ɫ�ı仯�� ��
��5���ζ����յ�ʱ����Һ��ɫ�ı仯�� ��
��6���ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ mL����Ʒ�Ĵ���Ϊ����Na2S2O3•5H2O��Է�������ΪM�� ��
III.Na2S2O3��Ӧ��
��7��Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO42‾�����������������÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
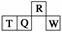 ������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ������ͼ��ʾ������T������������������������ȡ������жϲ���ȷ����
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ������ͼ��ʾ������T������������������������ȡ������жϲ���ȷ����
(����)
A�������̬�⻯������ȶ��ԣ�R > Q
B������������Ӧˮ��������ԣ�Q < W
C��ԭ�Ӱ뾶��T>Q>R
D����T������Һһ�� ������
������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A�� B��C��D��E���ֶ�����Ԫ�أ�A�ǵؿ��к�������Ԫ�أ�BԪ����3��ͬλ��B1��B2��B3��B3ԭ�ӵ���������B1��3����C�Ƿǽ�������ǿ��Ԫ�أ�D��C�����γ�DC�����ӻ���������ӵĵ��Ӳ�ṹ��ͬ��EԪ��ԭ�ӵ��������������ڲ����������6��������˵����ȷ���� (����)
B��C��D��E���ֶ�����Ԫ�أ�A�ǵؿ��к�������Ԫ�أ�BԪ����3��ͬλ��B1��B2��B3��B3ԭ�ӵ���������B1��3����C�Ƿǽ�������ǿ��Ԫ�أ�D��C�����γ�DC�����ӻ���������ӵĵ��Ӳ�ṹ��ͬ��EԪ��ԭ�ӵ��������������ڲ����������6��������˵����ȷ���� (����)
A��DԪ�صĽ������ڶ�����Ԫ������ǿ
B��E���ʳ������뵼����Ϻ��ά
C��A��Bֻ���γ�ԭ�Ӹ�����Ϊ1��2�Ļ�����
D������Ԫ�����γɵ�����������Ӧ��ˮ����������ǿ����C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����÷�Һ©�������һ��������( )
A���ƾ��͵� B��������Ȼ�̼ C�������ˮ D������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���ĽṹͲʽΪHO-CH2CH=CHCH2-COOH�����л��ﲻ���ܷ����Ļ�ѧ��Ӧ��( )
A��ˮ�ⷴӦ B��������Ӧ C���ӳɷ�Ӧ D��������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ΪԪ�����ڱ��е�һ���֣�����ѧʽ��Ԫ�ط����ش��������⡣
| �� ���� | I A | ��A | IIIA | ��A | VA | ��A | ��A | 0 |
| 2 | �� | �� |
| |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� | �� |
(1)11��Ԫ���У���ѧ��������õ��� ��
(2)�٢ڢݢޢ��У�����������ˮ�����м�����ǿ���� ������ǿ����
(3)�ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳���� �����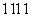 ����Ԫ���γɵ���̬�⻯�����ȶ����� ��
����Ԫ���γɵ���̬�⻯�����ȶ����� ��
(4)Ԫ�آ�����γɸ�����l��1������ĵ���ʽΪ�� ���û���������ʱ��ɫ Ϊ ���û����ﳣ���º�Ԫ�آߵ��⻯�ﷴӦ�����ӷ���ʽ�� ��
(5)д�����ʢݺ�Ԫ�آٵ�����������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽ��
��
(6) �ۺ͢���Ԫ�ؿ��γ� (����Ӽ������ۼ���)��д�����γɹ��̣�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ(D2O)����Ҫ�ĺ˹�ҵԭ�ϣ�����˵���������
A���(D)ԭ�Ӻ�����1������ B��1H��D����ͬλ��
C��H2O��D2O����ͬ�������� D��1H218O��D216O����Է���������ͬ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com