
·ÖĪö £Ø1£©Ģ¼ĖįÄĘŗĶŃĪĖį·“Ӧɜ³ÉĢ¼ĖįĒāÄĘŗĶĀČ»ÆÄĘ£¬µ±ŃĪĖį¹żĮæŹ±£¬Ģ¼ĖįĒāÄĘŗĶŃĪĖį·“Ӧɜ³ÉĀČ»ÆÄĘ”¢Ė®ŗĶ¶žŃõ»ÆĢ¼£¬¾Ż“ĖÅŠ¶ĻĢ¼ĖįĒāøłĄė×ÓÅØ¶ČµÄ±ä»Æ£»
£Ø2£©¢ŁŅ»ŌŖĖįÖŠ£¬ĒæĖįµÄÅضČŗĶĒāĄė×ÓÅضČĻąµČ£¬ČõĖįÖŠĖįµÄÅØ¶Č“óÓŚĒāĄė×ÓÅØ¶Č£»¶žŌŖĖįÖŠ£¬ĒāĄė×ÓÅضČĪŖĒæĖįÅØ¶ČµÄ2±¶£¬¾Ż“ĖČ·¶Ø3ÖÖĖįÖŠĪļÖŹµÄĮæÅضČ×ī“óµÄĖį£»
¢ŚpHĻąĶ¬µÄHClČÜŅŗ”¢H2SO4ČÜŅŗ”¢CH3COOHČÜŅŗÖŠ£¬“×ĖįŹĒČõµē½āÖŹ£¬ĖłŅŌ“×ĖįµÄÅضČ×ī“ó£¬ÖŠŗĶĻąĶ¬Ģå»żµÄČżÖÖĖį£¬“×ĖįŠčŅŖµÄĒāŃõ»ÆÄĘ×ī¶ą£»
¢Ū·“Ó¦ĖŁĀŹÓėĒāĄė×ÓÅØ¶Č³ÉÕż±Č£¬ĒāĄė×ÓÅضČŌ½“ó·“Ó¦ĖŁĀŹŌ½“ó£®
£Ø3£©øł¾Ż“æĖ®ÖŠc£ØOH-£©=c£ØH+£©£¬ČÜŅŗĻŌŹ¾ÖŠŠŌ½ųŠŠ·ÖĪö£»øł¾ŻøĆĪĀ¶ČĻĀ“æĖ®ÖŠĒāĄė×ÓÅضČŗĶĒāŃõøłĄė×ÓÅØ¶Č£¬¼ĘĖć³öĖ®µÄĄė×Ó»ż£¬øł¾ŻĖ®µÄĄė×Ó»ż¼ĘĖć³öČÜŅŗÖŠĒāŃõøłĄė×ÓµÄÅØ¶Č£®
½ā“š ½ā£ŗ£Ø1£©Ģ¼ĖįÄĘŗĶŃĪĖį·“Ӧɜ³ÉĢ¼ĖįĒāÄĘŗĶĀČ»ÆÄĘ£¬µ±ŃĪĖį¹żĮæŹ±£¬Ģ¼ĖįĒāÄĘŗĶŃĪĖį·“Ӧɜ³ÉĀČ»ÆÄĘ”¢Ė®ŗĶ¶žŃõ»ÆĢ¼£¬ĖłŅŌČÜŅŗÖŠĢ¼ĖįĒāøłĄė×ÓÅØ¶ČµÄ±ä»ÆŹĒ£ŗĻČŌö“óŗó¼õŠ”£¬¹ŹŃ”¢Ū£»
£Ø2£©¢ŁŅ»ŌŖĖįÖŠ£¬ĒæĖįµÄÅضČŗĶĒāĄė×ÓÅضČĻąµČ£¬ČõĖįÖŠĖįµÄÅØ¶Č“óÓŚĒāĄė×ÓÅØ¶Č£»¶žŌŖĖįÖŠ£¬ĒāĄė×ÓÅضČĪŖĒæĖįÅØ¶ČµÄ2±¶£¬ĖłŅŌpHĻąĶ¬µÄHClČÜŅŗ”¢H2SO4ČÜŅŗ”¢CH3COOHČÜŅŗÖŠĪļÖŹµÄĮæÅضČ×ī“óµÄŹĒ“×Ėį£¬¹Ź“š°øĪŖ£ŗ“×Ėį£»
¢ŚpHĻąĶ¬µÄHClČÜŅŗ”¢H2SO4ČÜŅŗ”¢CH3COOHČÜŅŗÖŠ£¬“×ĖįŹĒČõµē½āÖŹ£¬ĖłŅŌ“×ĖįµÄÅضČ×ī“ó£¬ÖŠŗĶĻąĶ¬Ģå»ż”¢ĻąĶ¬pHÖµµÄČżÖÖĖį£¬“×ĖįŠčŅŖµÄĒāŃõ»ÆÄĘ×ī¶ą£¬
¹Ź“š°øĪŖ£ŗCH3COOH£»
¢Ū·“Ó¦ĖŁĀŹÓėĒāĄė×ÓÅØ¶Č³ÉÕż±Č£¬ĒāĄė×ÓÅضČŌ½“ó·“Ó¦ĖŁĀŹŌ½“ó£¬pHĻąĶ¬µÄHClČÜŅŗ”¢H2SO4ČÜŅŗ”¢CH3COOHČÜŅŗÖŠĒāĄė×ÓÅضČĻąĶ¬£¬ĖłŅŌ·“Ó¦ĖŁĀŹĻąĶ¬£¬¹ŹŃ”D£®
£Ø3£©“æĖ®ĻŌŹ¾ÖŠŠŌ£¬c£ØOH-£©=c£ØH+£©=2”Į10-7mol/L£¬Ė®µÄĄė×Ó»żĪŖ£ŗ2”Į10-7”Į2”Į10-7=4”Į10-14£¬
ČÜŅŗÖŠĒāĄė×ÓÅضČc£ØH+£©=5.0”Į10-6mol/L£¬c£ØOH-£©=$\frac{4”Į1{0}^{-14}}{5”Į1{0}^{-6}}$=8”Į10-9mol/L£¬
¹Ź“š°øĪŖ£ŗ2”Į10-7mol/L£»8”Į10-9mol/L£®
µćĘĄ ±¾Ģāæ¼²éĮĖČõµē½āÖŹµÄµēĄė£¬Ć÷Č·ČõĖįÖŠĖįµÄÅضČŗĶĒāĄė×ÓÅØ¶ČµÄ¹ŲĻµŹĒ½ā±¾ĢāµÄ¹Ų¼ü£¬ÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ėę×Å»ÆѧæĘѧµÄ·¢Õ¹ŗĶĢįøߣ¬×ŌČ»½ēµÄŅ»ĒŠ¶¼½«ŅŌČĖµÄŅāÖ¾ĪŖ×ŖŅĘ | |
| B£® | ÉśĪļ¹ĢµŖŹĒÖøÖ²ĪļĶعżŅ¶ĆęÖ±½ÓĪüŹÕæÕĘųµÄµŖĘų | |
| C£® | ¹āµ¼ĻĖĪ¬ŹĒŅŌ¶žŃõ»Æ¹čĪŖÖ÷ŅŖŌĮĻÖĘ³ÉµÄ | |
| D£® | ĀĢÉ«Ź³Ę·ŹĒ²»ŗ¬ČĪŗĪ»ÆѧĪļÖŹµÄŹ³Ę· |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
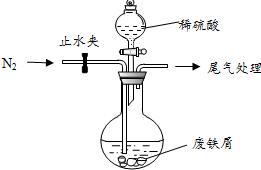 ĀĢ·Æ£ØFeSO4•7H2O£©ŹĒÖĪĮĘȱĢśŠŌʶŃŖµÄĢŲŠ§Ņ©£®Ä³»ÆѧŠĖȤŠ”×é¶ŌĀĢ·Æ½ųŠŠĮĖČēĻĀµÄĢ½¾æ£ŗ
ĀĢ·Æ£ØFeSO4•7H2O£©ŹĒÖĪĮĘȱĢśŠŌʶŃŖµÄĢŲŠ§Ņ©£®Ä³»ÆѧŠĖȤŠ”×é¶ŌĀĢ·Æ½ųŠŠĮĖČēĻĀµÄĢ½¾æ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 68.76 kJ | B£® | 57.3 kJ | C£® | 34.38 kJ | D£® | 17.19 kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ¼ÓČČĒ°ÖŹĮæ | ¼ÓČČŗóÖŹĮæ | |
| W1£ØČŻĘ÷£© | W2£ØČŻĘ÷+¾§Ģ壩 | W3£ØČŻĘ÷+ĪŽĖ®ĮņĖįĶ£© |
| 5.4g | 7.9g | 6.8g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆNaOHČÜŅŗĪüŹÕÉŁĮæCO2 CO2+OH-=CO32-+H2O | |
| B£® | ŃĪĖįµĪŌŚĶʬÉĻ Cu+2H+=Cu2++H2O | |
| C£® | H2SO4ÓėBa£ØOH£©2ČÜŅŗ·“Ó¦ Ba2++2OH-+2H++SO42-=BaSO4”ż+2H2O | |
| D£® | CaCl2ČÜŅŗÖŠĶØČėCO2 Ca2++CO2+H2O=CaCO3”ż+2H+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | O2ČŌČ»ĪŖ 1mol | B£® | ·“Ó¦×ć¹»³¤Ź±¼äŗó£¬ĖŁĀŹ±äĪŖĮć | ||
| C£® | SO2”¢O2ŗĶ SO3 Ķ¬Ź±“ęŌŚ | D£® | SO2ĶźČ«×Ŗ»ÆĪŖ SO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com