
������ʵ������У����ֵġ��쳣������Ľ��ͣ�����ȷ����
| A��������Ͷ�뵽����ͭ��Һ�У����ɺ�ɫ���������Ϊ������ͭ������ |
| B���������Ƽ��뵽���з�̪��ˮ�У���Һ����ٱ���ɫ��������ǿ�������������� |
| C�������Ķ�����̼ͨ�����ʯ��ˮ�У���Һ������ٱ���壬˵���������ɿ�������ˮ���� |
| D�����Ȼ���������Һ�У��μ�����������Һ �����а�ɫ�������������ɫ�ٱ���ɫ��˵�������������ױ������е��������� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| B������� | �� | �� | �� | �� |
| ��պ�Լ� | ʯ����Һ | Ʒ����Һ | ���ۺ͵�ˮ���Һ | ������ |
| ���� | ��ɫ | dz��ɫ | ||
| ����SO2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
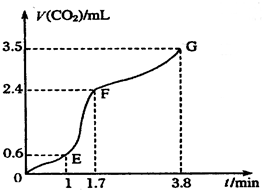 ��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺
��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com