
��֪25��ʱ�������ʵĵ����(��Һ��Ũ��Ϊ0.1mol/L)���±���

(1)25��ʱ��0.1mol/L����������Һ��[H+]�ɴ�С��˳����________��
(2)25��ʱ��pHֵ��ͬ������������Һ�����ʵ���Ũ���ɴ�С��˳����________��
(3)25��ʱ��������Zn�۷���������pH��1��������Һ�У�����H2�����(ͬ��ͬѹ��)�ɴ�С��˳����________��
(4)25��ʱ��0.1mol/L H2SO4��Һ��HSO4���ĵ����С��NaHSO4��Һ��HSO4���ĵ���ȵ�ԭ����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��������Һ | ������������Һ | �۴�����Һ | ������ |
| HSO4-?H++SO42- | HSO4-?H++SO42- | CH3COOH?CH3COO-+H+ | HCl=H++Cl- |
| 10% | 29% | 1.33% | 100% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���֪25��ʱ�������ʵĵ����(��ҺŨ�Ⱦ�Ϊ0.1mol/L) ���±�(��֪����ĵ�һ����������ȫ��):
| ��������Һ | ������������Һ | �۴�����Һ | ������ |
| HSO4�� | HSO4�� | CH3COOH | HCl = H+��Cl�� |
| 10�G | 29�G | 1.33�G | 100�G |
�Ÿ������⣬��д����������Һ�еμ���������������Һ��Ӧ�����ӷ�Ӧ����ʽ
��25��ʱ��0.1mol/L����������Һ��C(H+)�ɴ�С��˳���� �������,��ͬ)��
��25��ʱ��������п�۷�������pH=1������������Һ�У�����H2���������ͬ״�����ɴ�С��˳���� ��
��25��ʱ��0.1mol/L H2SO4�е�HSO4���ĵ����С��0.1mol/L NaHSO4��HSO4���ĵ���ȵ�ԭ���ǣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ��һ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��8�֣���֪25��ʱ�������ʵĵ����(��ҺŨ�Ⱦ�Ϊ0.1mol/L) ���±�(��֪����ĵ�һ����������ȫ��):
| ��������Һ | ������������Һ | �۴�����Һ | ������ |
HSO4��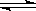 H+��SO42�� H+��SO42�� | HSO4��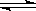 H+��SO42�� H+��SO42�� | CH3COOH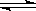 CH3COO��+H+ CH3COO��+H+ | HCl = H+��Cl�� |
| 10�G | 29�G | 1.33�G | 100�G |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ���ѡ�ޣ��������棩 ���ͣ������
����ȱ�ʾ����ʵ����ǿ��������ȵĶ��壺
�������ѵ���ĵ���ʷ���������Һ��ԭ�е���ʵ��ܷ���������100%��
��֪25��ʱ��������(��)�ĵ����(��ҺŨ�Ⱦ�Ϊ0.1 mol��L��1)���±���
��� ����(��) ����Ȧ�
A ������Һ(��һ����ȫ����)�� �ڶ��� HSO4- H����SO42- 10%
H����SO42- 10%
B ����������Һ�� HSO4- H����SO42 29%
H����SO42 29%
C ��� CH3COOH CH3COO����H�� 1.33%
CH3COO����H�� 1.33%
D ��� HCl��H����Cl�� 100%
��1��25��ʱ��0.1 mol��L��1����������Һ�У�c(H��)�Ӵ�С��˳���� (�����)��
��2��25��ʱ��0.1 mol��L��1������Һ��HSO4-�ĵ����С����ͬ�¶���0.1 mol��L��1��������
��Һ��HSO4-�ĵ���ȣ���ԭ���� ��
��3������ĵ���ƽ�ⳣ��K�ı���ʽ�� ������ĵ���ƽ�ⳣ��
K�����Ȧ��Ĺ�ϵʽΪ��K= ���ú����Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣���֪25��ʱ�������ʵĵ����(��ҺŨ�Ⱦ�Ϊ0.1mol/L) ���±�(��֪����ĵ�һ����������ȫ��):
|
��������Һ |
������������Һ |
�۴�����Һ |
������ |
|
HSO4�� |
HSO4�� |
CH3COOH |
HCl = H+��Cl�� |
|
10�G |
29�G |
1.33�G |
100�G |
�Ÿ������⣬��д����������Һ�еμ���������������Һ��Ӧ�����ӷ�Ӧ����ʽ
��25��ʱ��0.1mol/L����������Һ��C(H+)�ɴ�С��˳���� �������,��ͬ)��
��25��ʱ��������п�۷�������pH=1������������Һ�У�����H2���������ͬ״�����ɴ�С��˳���� ��
��25��ʱ��0.1mol/L H2SO4�е�HSO4���ĵ����С��0.1mol/L NaHSO4��HSO4���ĵ���ȵ�ԭ���ǣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com