
 ����2�֣������ԣ����ۼ�����1�֣� S=C=S����2�֣�����5�֣�
����2�֣������ԣ����ۼ�����1�֣� S=C=S����2�֣�����5�֣�

 ��ѧ����ϵ�д�
��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
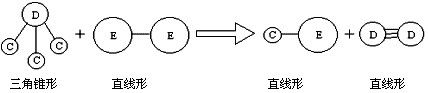
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ڱ��н����ͷǽ����ķֽ紦Ѱ�Ҵ��������¡���ʴ�ĺϽ���� |
| B��Ԫ���������ź˵�����ĵ������������Եı仯��Ԫ�������� |
| C����ɫֲ����й������ʱ����̫����ת��Ϊ��ѧ�ܡ����桱���� |
| D����Է���������ͬ�����ṹ��ͬ�Ļ����ﻥ��Ϊͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ԫ�ص�ԭ���������Ӹ���Ϊ2 | B��Ԫ�ص�ԭ���������Ӹ���Ϊ6 |
| C��λ��Ԫ�����ڱ��Т�A���ұߵ�Ԫ�� | D��������RO4����RԪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��RO5�� | B��RO3�� | C��RO42�� | D��RO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��OH-��NH4+ | B��H2O��NH3 | C��Na+ ��OH- | D��O2-��NH4+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com